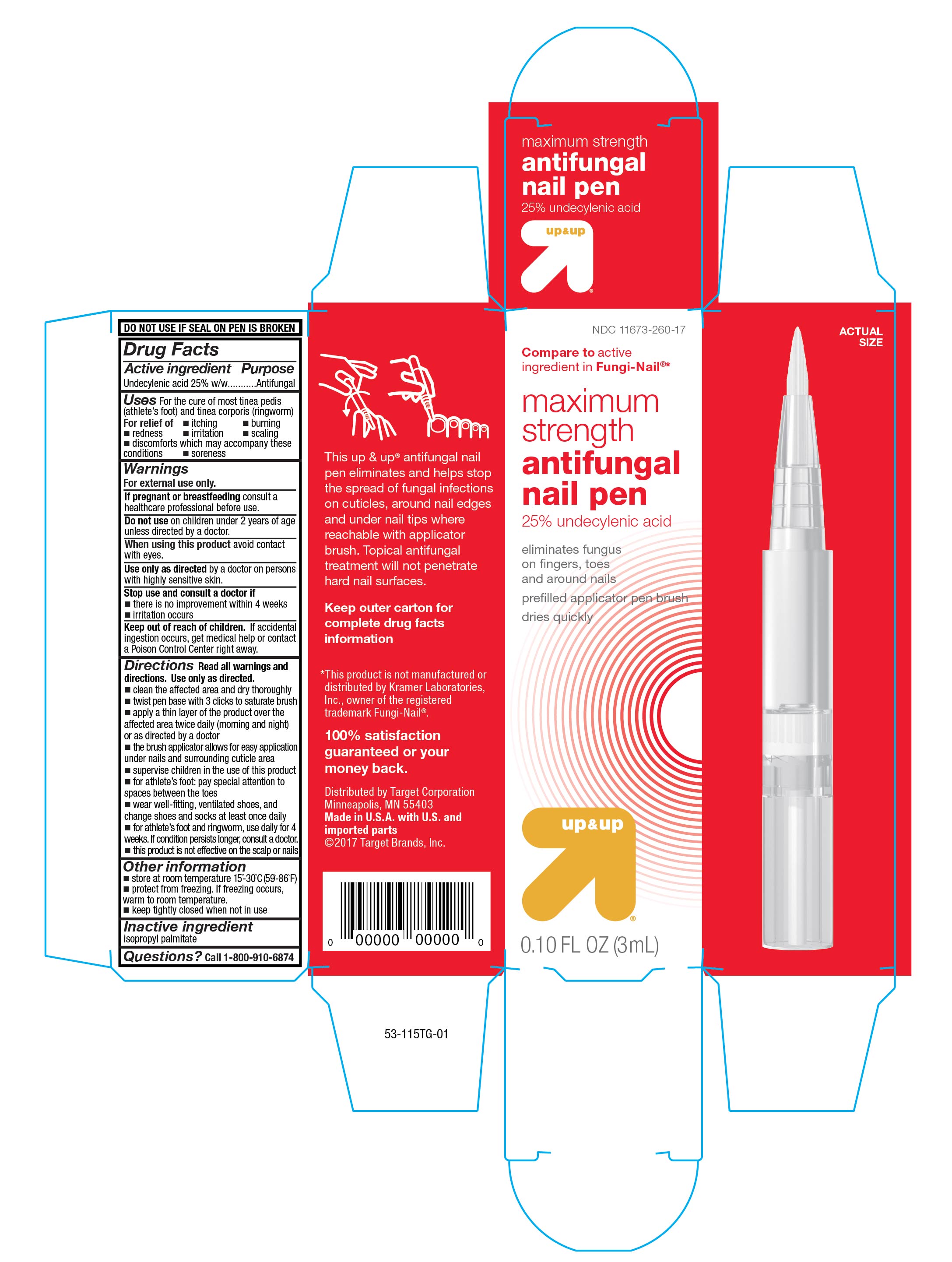
UNDECYLENIC ACID- maximum strength antifungal pen liquid
Target Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Maximum Strength Antifungal Pen
Uses
For cure of most tinea pedis (athlete's foot) and tinea corporis (ringworm).
For relief of:
- itching
- burning
- redness
- irritation
- scaling
- soreness
- discomfort which may accompany these conditions
Directions
Read all warnings and directions. Use only as directed.
- clean the affected area and dry thoroughly
- twist pen base with 3 clicks to saturate brush
- apply a thin layer of the product over the affecter area twice daily (morning and night) or as directed by a doctor
- the brush applicator allows for easy application under nails and surrounding cuticle area
- supervise children in the use of this product
- for athlete's foot: pay special attention to spaces between toes
- wear well-fitting, ventilated shoes, and change shoes and socks at least once daily
- for athlete's foot and ringworm, use daily for 4 weeks. If conditions persists longer, consult a doctor
- this product is not effective on the scalp or nails
Other information
- store at room temperature 15°-30°C (59° - 86°F)
- protect from freezing. If freezing occurs warm to room temperature
- keep tightly closed when not in use
Principal Display Panel
Front Panel
Compare to active ingredient in Fungi-Nail ®*
maximum strength
antifungal pen
25% undecylenic acid
eliminates fungus on fingers, toes and around nails
pre-filled applicator pen brush
dries quickly
up&up
0.10 FL OZ (3mL)
Side Panel
This up & up ® antifungal nail pen eliminates and helps stop the spread of fungal infections on cuticles, around nail edges and under nail tips where reachable with applicator brush. Topical antifungal treatment will not penetrate hard nail surfaces.
Keep outer carton for complete drug facts information
*This product is not manufactured or distributed by Kramer Laboratories, Inc., owner of the registered trademark Fungi-Nail ®.
100% satisfaction guarantreed or your money back.

| UNDECYLENIC ACID
maximum strength antifungal pen liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Target Corporation (006961700) |