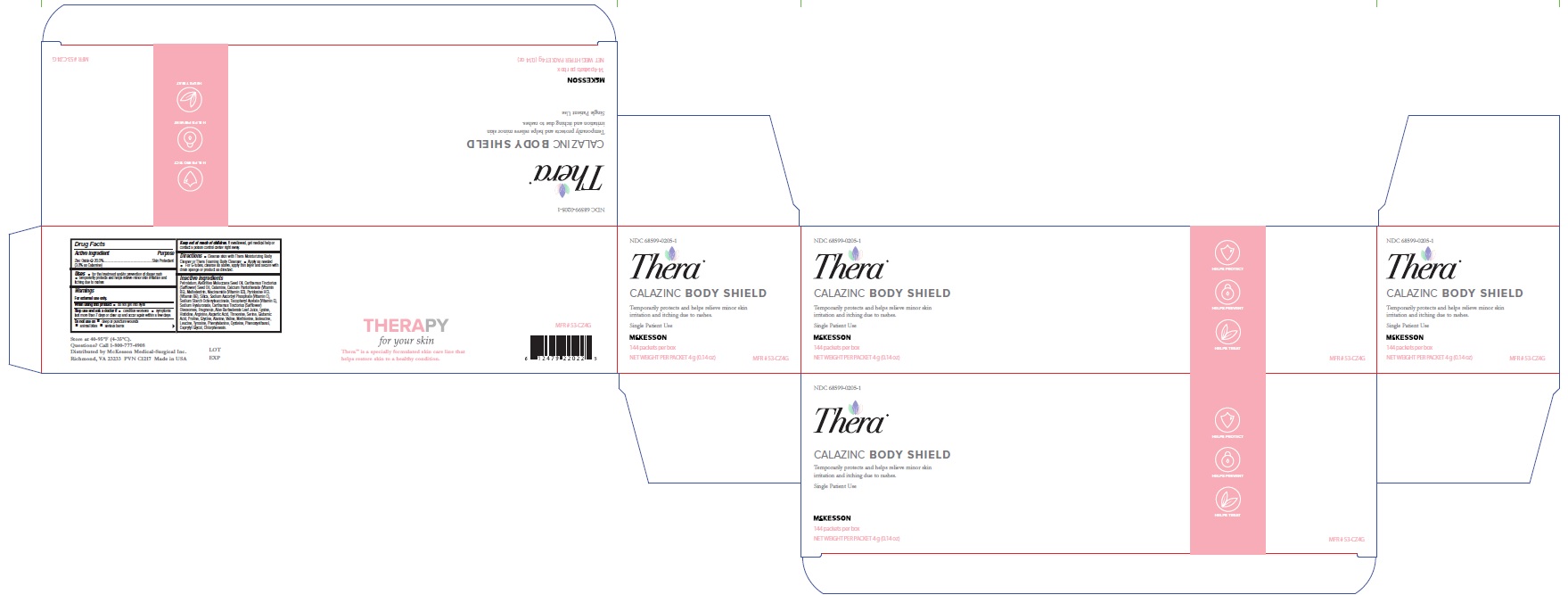
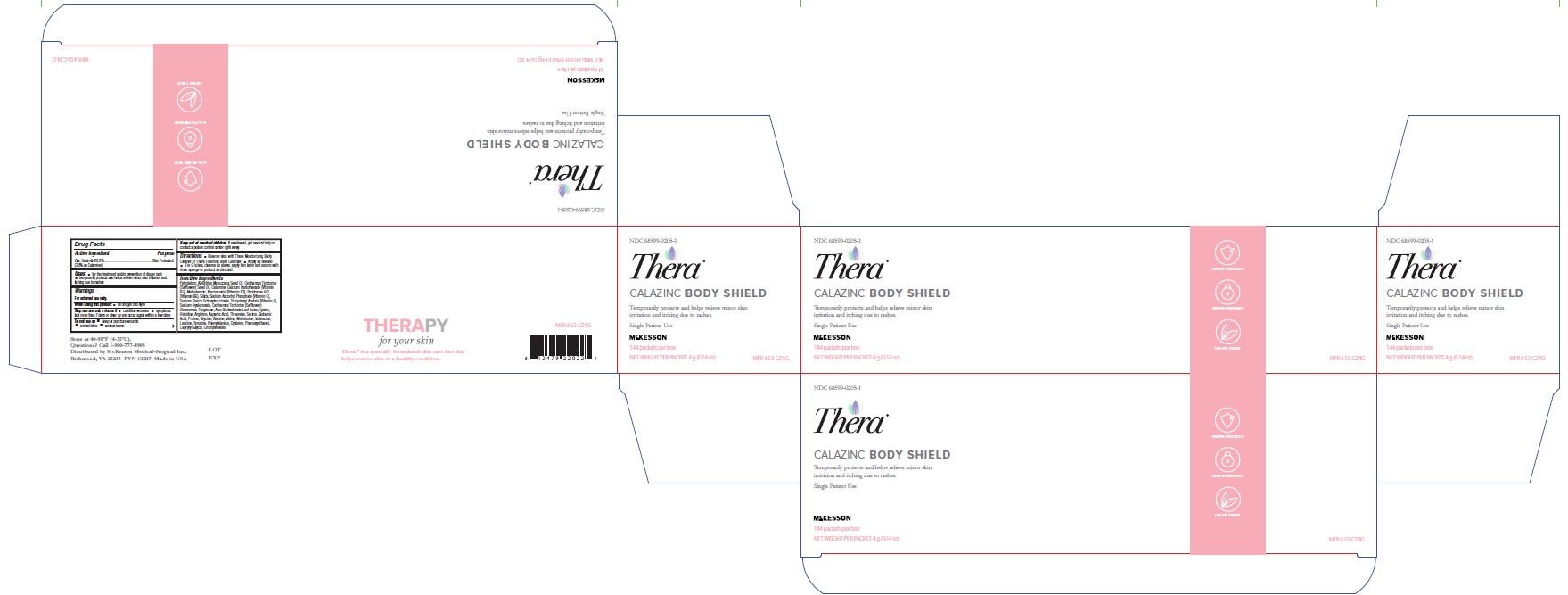
Label: THERA CALAZINC BODY SHIELD- zinc oxide paste
- NDC Code(s): 68599-0205-1, 68599-0205-4
- Packager: McKesson Medical-Surgical Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 17, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Keep out of reach of children.
- Uses
- Warnings
- Directions
- Other information
-
INACTIVE INGREDIENT
- Aleurites Moluccana Seed Oil,
- Aloe Barbadensis (Aloe Vera) Leaf Juice,
- SAFFLEX TM (Consisting of: Calcium Pantothenate (Vitamin B 5),
- Maltodexdrin,
- Niacinamide (Vitamin B 3),
- Pyridoxine HCl (Vitamin B 6),
- Silica,
- Sodium Ascorbyl Phosphate (Vitamin C),
- Sodium Starch Octenylsuccinate,
- Tocopheryl Acetate (Vitamin E)), Bisabolol,
- Carthamus Tinctorius (Safflower) Olesomes,
- Carthamus Tinctorius (Safflower) Seed Oil,
- Lavender Ylang Fragrance,
- Modified Corn Starch,
- Pentaerythrityl Tetra-di-t-Butyl Hydroxyhydrocinnamate,
- Petrolatum,
- Phenoxyethanol,
- Sodium Hyaluronate,
- Zingiber Officinale (Ginger) Root Extract.
- Label (4 oz)
- Label(4 g)
- Box
-
INGREDIENTS AND APPEARANCE
THERA CALAZINC BODY SHIELD
zinc oxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68599-0205 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 200 mg in 1 g Inactive Ingredients Ingredient Name Strength KUKUI NUT OIL (UNII: TP11QR7B8R) ALOE VERA LEAF (UNII: ZY81Z83H0X) CALCIUM PANTOTHENATE (UNII: 568ET80C3D) MALTODEXTRIN (UNII: 7CVR7L4A2D) NIACINAMIDE (UNII: 25X51I8RD4) PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) LEVOMENOL (UNII: 24WE03BX2T) SAFFLOWER OIL (UNII: 65UEH262IS) PETROLATUM (UNII: 4T6H12BN9U) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GINGER (UNII: C5529G5JPQ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68599-0205-4 113 g in 1 TUBE; Type 0: Not a Combination Product 09/01/2011 2 NDC:68599-0205-1 144 in 1 CARTON 09/01/2011 2 4 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 09/01/2011 Labeler - McKesson Medical-Surgical Inc. (023904428) Establishment Name Address ID/FEI Business Operations Central Solutions 007118524 manufacture(68599-0205)