ACETAMINOPHEN, CHLORPHENIRAMINE MALEATE, PHENYLEPHRINE HYDROCHLORIDE- acetaminophen, chlorpheniramine maleate, and phenylephrine hydrochloride tablet, coated
AAA Pharmaceutical, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
RES - 1128 - 2019-1012
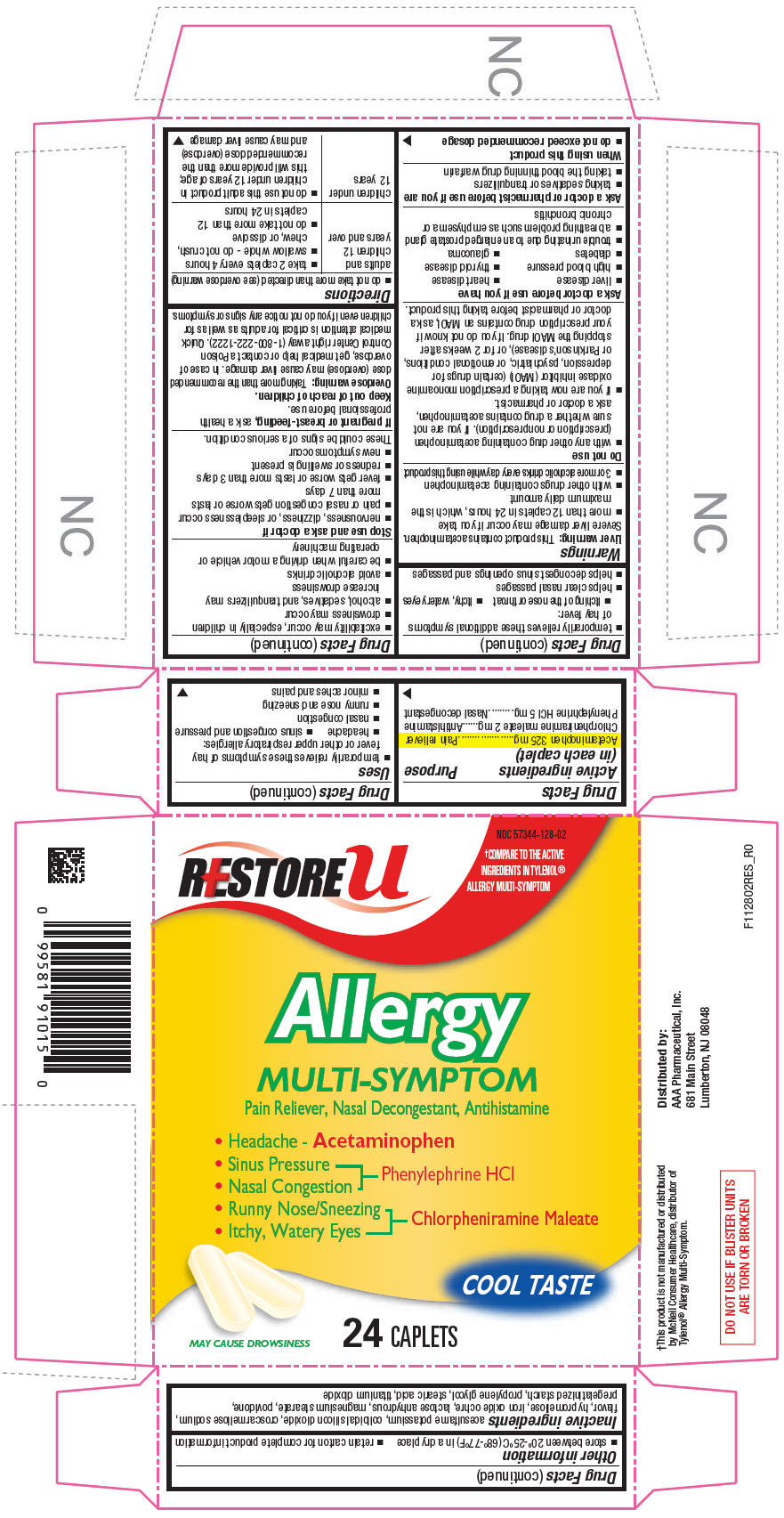
Uses
- temporarily relieves these symptoms of hay fever or other upper respiratory allergies:
- headache
- sinus congestion and pressure
- nasal congestion
- runny nose and sneezing
- minor aches and pains
- temporarily relieves these additional symptoms of hay fever:
- itching of the nose or throat
- itchy, watery eyes
- helps clear nasal passages
- helps decongest sinus openings and passages
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 12 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- do not exceed recommended dosage
- excitability may occur, especially in children
- drowsiness may occur
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic drinks
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- pain or nasal congestion gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
These could be signs of a serious condition.
Overdose warning
Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- do not take more than directed (see overdose warning)
| adults and children 12 years and over |
|
| children under
12 years |
|
Other information
- store between 20°-25°C (68°-77°F) in a dry place
- retain carton for complete product information
Inactive ingredients
acesulfame potassium, colloidal silicon dioxide, croscarmellose sodium, flavor, hypromellose, iron oxide ochre, lactose anhydrous, magnesium stearate, povidone, pregelatinized starch, propylene glycol, stearic acid, titanium dioxide
PRINCIPAL DISPLAY PANEL - 24 Caplet Blister Pack Carton
NDC 57344-128-02
RESTORE u
†COMPARE TO THE ACTIVE
INGREDIENTS IN TYLENOL®
ALLERGY MULTI-SYMPTOM
Allergy
MULTI-SYMPTOM
Pain Reliever, Nasal Decongestant, Antihistamine
- Headache - Acetaminophen
- Sinus Pressure
- Nasal Congestion
Phenylephrine HCl - Runny Nose/Sneezing
- Itchy, Watery Eyes
Chlorpheniramine Maleate
COOL TASTE
MAY CAUSE DROWSINESS
24 CAPLETS
| ACETAMINOPHEN, CHLORPHENIRAMINE MALEATE, PHENYLEPHRINE HYDROCHLORIDE
acetaminophen, chlorpheniramine maleate, and phenylephrine hydrochloride tablet, coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - AAA Pharmaceutical, Inc. (181192162) |