KERASAL NEUROCREAM - capsaicin, camphor cream
MOBERG PHARMA NORTH AMERICA LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
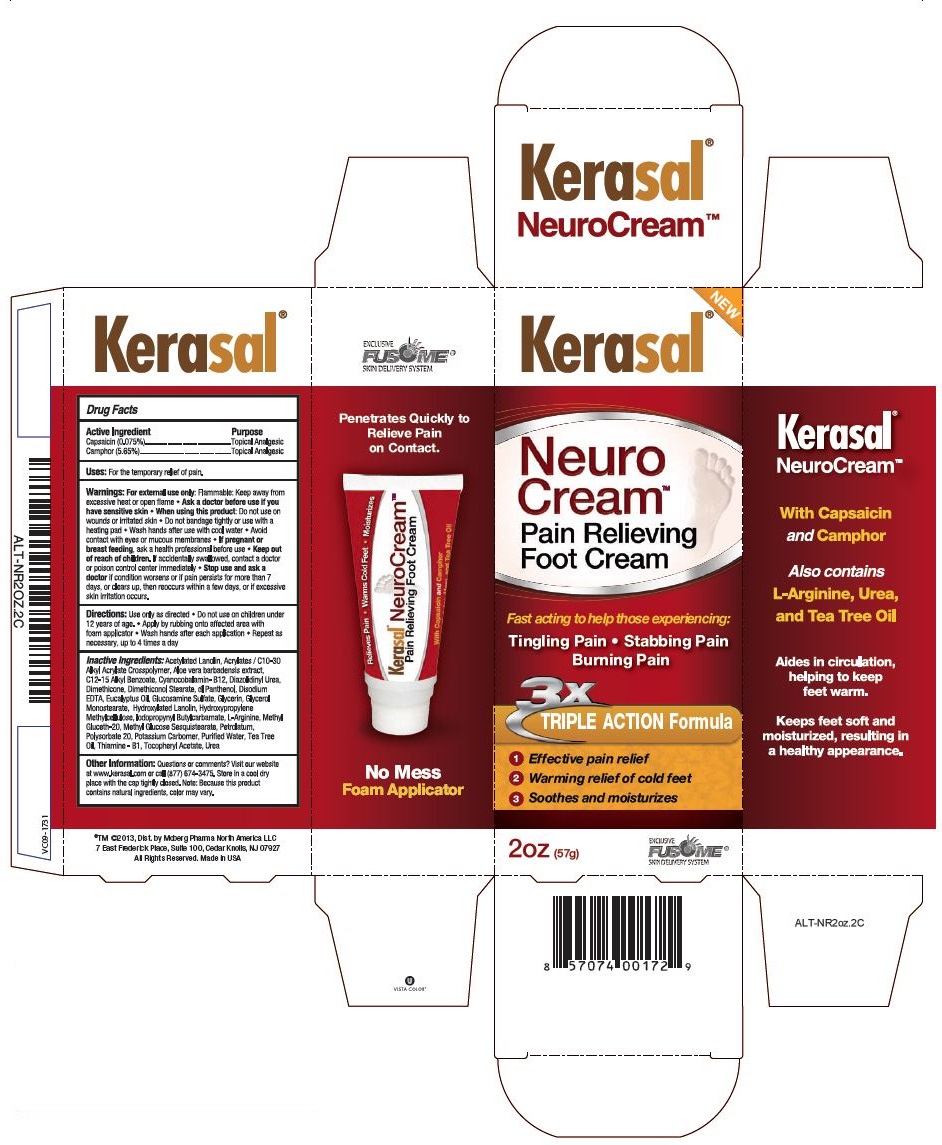
Kerasal NeuroCream™
Drug Facts
Warnings:
For external use only:
- Flammable: Keep away from excessive heat or open flame
- Ask a doctor before use if you have sensitive skin
When using this product:
- Do not use on wounds or irritated skin
- Do not bandage tightly or use with a heating pad
- Wash hands after use with cool water
- Avoid contact with eyes or mucous membranes
Keep out of reach of children.
If accidentally swallowed, contact a doctor or poison control center immediately.
Stop use and ask a doctor
if condition worsens or if pain persists for more than 7 days, or clears up, then reoccurs within a few days, or if excessive skin irritation occurs.
Directions
- Use only as directed
- Do not use on children under 12 years of age.
- Apply by rubbing onto affected area with foam applicator
- Wash hands after each application
- Repeat as necessary, up to 4 times a day
Inactive Ingredients
Acetylated Lanolin, Acrylates / C10-30 Alkyl Acrylate Crosspolymer, Aloe vera barbadensis extract, C12-15 Alkyl Benzoate, Cyanocobalamin - B12, Diazolidinyl Urea, Dimethicone, Dimethiconol Stearate, dl Panthenol, Disodium EDTA, Eucalyptus Oil, Glucosamine Sulfate, Glycerin, Glycerol Monostearate, Hydroxylated Lanolin, Hydroxypropylene Methylcellulose, Iodopropynyl Butylcarbamate, L-Arginine, Methyl Gluceth-20, Methyl Glucose Sesquistearate, Petrolatum, Polysorbate 20, Potassium Carbomer, Purified Water, Tea Tree Oil, Thiamine - B1, Tocopheryl Acetate, Urea
Other Information:
Questions or comments? Visit our website at www.kerasal.com or call (877) 674-3475. Store in a cool dry place with the cap tightly closed. Note: Because this product contains natural ingredients, color may vary.
Principal Display Label
NEW
Kerasal® NeuroCream™
Pain Relieving Foot Cream
Fast acting to help those experiencing:
Tingling Pain • Stabbing Pain • Burning Pain
3X TRIPLE ACTION Formula
1 Effective pain relief
2 Warming relief of cold feet
3 Soothes and moisturizes
2oz (57g)
EXCLUSIVE FUSOME®
SKIN DELIVERY SYSTEM
Kerasal® NeuroCream™
With Capsaicin and Camphor
Also contains
L-Arginine, Urea, and Tea Tree Oil
Aides in circulation, helping to keep feet warm.
Keeps feet soft and moisturized, resulting in a healthy appearance.
Penetrates Quickly to Relieve Pain on Contact.
No Mess
Foam Applicator
ALT-NR2OZ.2C
®TM ©2013, Dist. By Moberg Pharma North America LLC
7 East Frederick Place, Suite 100, Cedar Knolls, NJ 07927
All Rights Reserved. Made in USA
Relieves Pain • Warms Cold Feet • Moisturizes
Kerasal® NeuroCream™
Pain Relieving Foot Cream
With Capsaicin and Camphor
Also contains: L-Argine, Urea, and Tea Tree Oil
EXCLUSIVE
FUSOME®
SKIN DELIVERY SYSTEM
2oz (57g)
®TM ©2013, Dist. by Moberg Pharma North America LLC, 7 East Frederick Place,
Cedar Knolls, NJ 07927 All Rights Reserved. Made in USA. ALT-NR2oz.2T

| KERASAL NEUROCREAM
capsaicin, camphor cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - MOBERG PHARMA NORTH AMERICA LLC (192527062) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Smart Science | 035907919 | MANUFACTURE(16864-013) | |