ANTIBACTERIAL AMBER FLORAL- antibacterial amber floral hand soap liquid
Theochem Laboratories Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
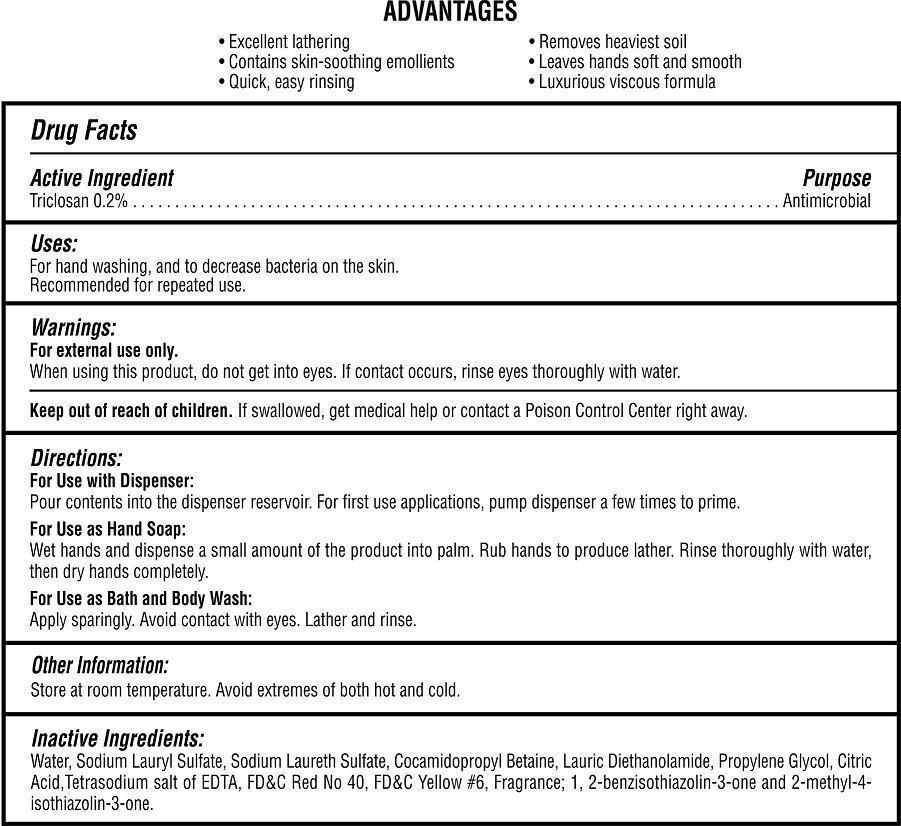
Antibacterial Amber Floral Hand Soap
Warnings:
For external use only.
When using this product, do not get into eyes. If contact occurs, rinse eyes thoroughly with water.
Keep out of reach of children:
If swallowed, get medical help or contact a Poison Control Center right away.
Directions:
For Use with Dispenser: Pour contents into the dispenser reservoir. For first use applications, pump dispenser a few times to prime.
For Use as Hand Soap: Wet hands and dispense a small amount of the product into palm. Rub hands to produce lather. Rinse thoroughly with water, then dry hands completely.
For Use as Bath and Body Wash: Apply sparingly. Avoid contact with eyes. Lather and rinse.
| ANTIBACTERIAL AMBER FLORAL
antibacterial amber floral hand soap liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Theochem Laboratories Inc (004100038) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Theochem Laboratories Inc | 004100038 | manufacture(68878-115) | |