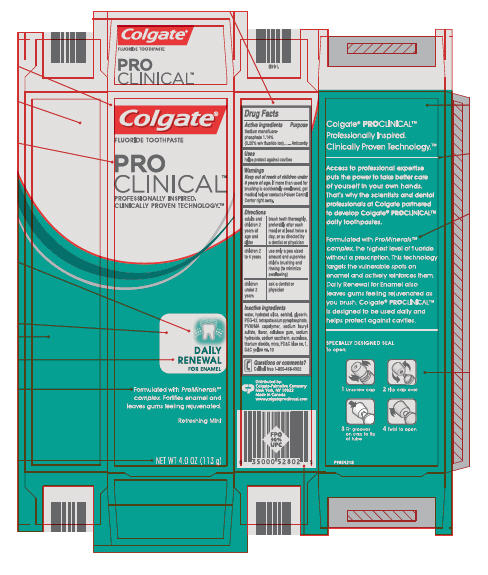
COLGATE PROCLINICAL REFRESHING MINT- sodium monofluorophosphate paste, dentifrice
Colgate-Palmolive Canada
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Colgate®
FLUORIDE TOOTHPASTE
Directions
| adults and children 2 years of age and older | brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician |
| children 2 to 6 years | use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) |
| children under 2 years | ask a dentist or physician |
| COLGATE PROCLINICAL REFRESHING MINT
sodium monofluorophosphate paste, dentifrice |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Colgate-Palmolive Canada (259157246) |
Revised: 9/2019
Document Id: ef3c1b95-68e1-4b37-bfa8-405b27f1acf2
Set id: 95e261e5-a8b4-462a-b484-4fcbf6e55648
Version: 3
Effective Time: 20190910
Colgate-Palmolive Canada