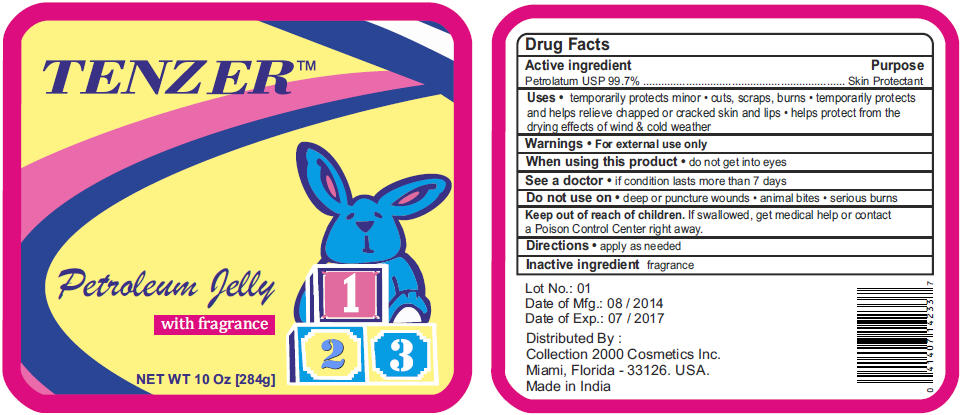
Label: TENZER BABY- petrolatum jelly
-
Contains inactivated NDC Code(s)
NDC Code(s): 69174-125-10, 69174-125-13 - Packager: Collection 2000
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 5, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredient
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 284 g Container Label
-
INGREDIENTS AND APPEARANCE
TENZER BABY
petrolatum jellyProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69174-125 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 99.7 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69174-125-10 284 g in 1 CONTAINER; Type 0: Not a Combination Product 2 NDC:69174-125-13 368 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part347 05/30/2012 Labeler - Collection 2000 (809478688) Establishment Name Address ID/FEI Business Operations Vikshara Trading & Investments Ltd. 675920690 MANUFACTURE(69174-125)