BACTROBAN- mupirocin calcium cream
GlaxoSmithKline LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BACTROBAN Cream safely and effectively. See full prescribing information for BACTROBAN Cream.
BACTROBAN (mupirocin) cream, for topical use Initial U.S. Approval: 1997 INDICATIONS AND USAGEBACTROBAN cream is an RNA synthetase inhibitor antibacterial indicated for the treatment of secondarily infected traumatic skin lesions (up to 10 cm in length or 100 cm2 in area) due to susceptible isolates of Staphylococcus aureus and Streptococcus pyogenes. (1) DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSCream: 20 mg (2% w/w) of mupirocin per gram in 15-gram and 30-gram tubes. (3) CONTRAINDICATIONSKnown hypersensitivity to mupirocin or any of the excipients of BACTROBAN cream. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most frequent adverse reactions (at least 1%) were headache, rash, and nausea. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 2/2020 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
BACTROBAN cream is indicated for the treatment of secondarily infected traumatic skin lesions (up to 10 cm in length or 100 cm2 in area) due to susceptible isolates of Staphylococcus aureus (S. aureus) and Streptococcus pyogenes (S. pyogenes).
2 DOSAGE AND ADMINISTRATION
- •
- For Topical Use Only.
- •
- Apply a small amount of BACTROBAN cream, with a cotton swab or gauze pad, to the affected area 3 times daily for 10 days.
- •
- Cover the treated area with gauze dressing if desired.
- •
- Re-evaluate patients not showing a clinical response within 3 to 5 days.
- •
- BACTROBAN cream is not for intranasal, ophthalmic, or other mucosal use [see Warnings and Precautions (5.2, 5.6)].
- •
- Do not apply BACTROBAN cream concurrently with any other lotions, creams or ointments [see Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
BACTROBAN cream is a white cream that contains 20 mg (2% w/w) of mupirocin per gram in an oil- and water‑based emulsion, supplied in 15-gram and 30-gram tubes.
4 CONTRAINDICATIONS
BACTROBAN cream is contraindicated in patients with known hypersensitivity to mupirocin or any of the excipients of BACTROBAN cream.
5 WARNINGS AND PRECAUTIONS
5.1 Severe Allergic Reactions
Systemic allergic reactions, including anaphylaxis, urticaria, angioedema, and generalized rash, have been reported in patients treated with formulations of BACTROBAN, including BACTROBAN cream [see Adverse Reactions (6.2)].
5.2 Eye Irritation
Avoid contact with the eyes. In case of accidental contact, rinse well with water.
5.3 Local Irritation
In the event of a sensitization or severe local irritation from BACTROBAN cream, usage should be discontinued, and appropriate alternative therapy for the infection instituted.
5.4 Clostridium difficile-Associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- •
- Severe Allergic Reactions [see Warnings and Precautions (5.1)]
- •
- Eye Irritation [see Warnings and Precautions (5.2)]
- •
- Local Irritation [see Warnings and Precautions (5.3)]
- •
- Clostridium difficile-Associated Diarrhea [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In 2 randomized, double‑blind, double‑dummy trials, 339 subjects were treated with topical BACTROBAN cream plus oral placebo. Adverse reactions occurred in 28 (8.3%) subjects. The following adverse reactions were reported by at least 1% of subjects in connection with the use of BACTROBAN cream in clinical trials: headache (1.7%), rash (1.1%), and nausea (1.1%).
Other adverse reactions which occurred in less than 1% of subjects were: abdominal pain, burning at application site, cellulitis, dermatitis, dizziness, pruritus, secondary wound infection, and ulcerative stomatitis.
In a supportive trial in the treatment of secondarily infected eczema, 82 subjects were treated with BACTROBAN cream. The incidence of adverse reactions was as follows: nausea (4.9%), headache and burning at application site (3.6% each), pruritus (2.4%), and 1 report each of abdominal pain, bleeding secondary to eczema, pain secondary to eczema, hives, dry skin, and rash.
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following reactions have been identified during postmarketing use of BACTROBAN cream. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal relationship to BACTROBAN cream.
Immune System Disorders
Systemic allergic reactions, including anaphylaxis, urticaria, angioedema, and generalized rash [see Warnings and Precautions (5.1)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are insufficient human data to establish whether there is a drug-associated risk with BACTROBAN cream in pregnant women. Systemic absorption of mupirocin through intact human skin is minimal following topical administration of BACROBAN cream [see Clinical Pharmacology (12.3)]. No developmental toxicity was observed in rats or rabbits treated with mupirocin subcutaneously during organogenesis at doses of 160 or 40 mg per kg per day, respectively (22 and 11 times the human topical dose based on calculations of dose divided by the entire body surface area).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. The estimated background risk in the U.S. general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Data
Animal Data: Developmental toxicity studies have been performed with mupirocin administered subcutaneously to rats and rabbits at doses up to 160 mg per kg per day during organogenesis. This dose is 22 and 43 times, respectively, the human topical dose (approximately 60 mg mupirocin per day) based on calculations of dose divided by the entire body surface area. Maternal toxicity was observed (body weight loss/decreased body weight gain and reduced feeding) in both species with no evidence of developmental toxicity in rats. In rabbits, excessive maternal toxicity at the high dose precluded the evaluation of fetal outcomes. There was no developmental toxicity in rabbits at 40 mg per kg per day, 11 times the human topical dose based on calculations of dose divided by the entire body surface area.
Mupirocin administered subcutaneously to rats in a pre- and postnatal development study (dosed during late gestation through lactation) was associated with reduced offspring viability in the early postnatal period at a dose of 106.7 mg per kg, in the presence of injection site irritation and/or subcutaneous hemorrhaging. This dose is 14 times the human topical dose based on calculations of dose divided by the entire body surface area. The no-observed adverse effect level in this study was 44.2 mg per kg per day, which is 6 times the human topical dose.
8.2 Lactation
Risk Summary
It is not known whether mupirocin is present in human milk, has effects on the breastfed child, or has effects on milk production. However, breastfeeding is not expected to result in exposure of the child to the drug due to the minimal systemic absorption of mupirocin in humans following topical administration of BACTROBAN cream [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for BACTROBAN cream and any potential adverse effects on the breastfed child from BACTROBAN cream or from the underlying maternal condition.
Clinical Considerations
To minimize oral exposure of the drug to children, a breast and/or nipple being treated with BACTROBAN cream should be thoroughly washed prior to breastfeeding.
8.4 Pediatric Use
The safety and effectiveness of BACTROBAN cream have been established in the age-groups 3 months to 16 years. Use of BACTROBAN cream in these age-groups is supported by evidence from adequate and well‑controlled trials of BACTROBAN cream in adults with additional data from 93 pediatric subjects studied as part of the pivotal trials in adults [see Clinical Studies (14)].
11 DESCRIPTION
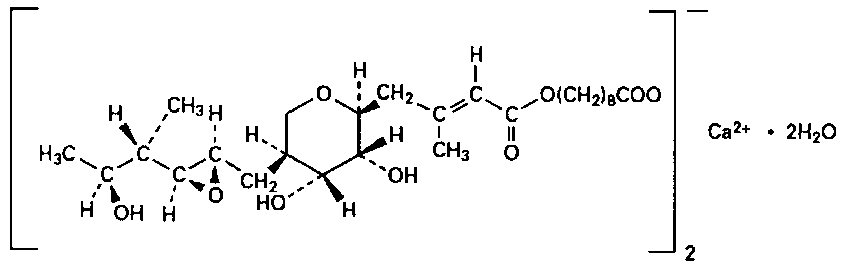
BACTROBAN (mupirocin) cream, 2% contains the dihydrate crystalline calcium hemi-salt of the RNA synthetase inhibitor antibacterial, mupirocin. Chemically, it is (αE,2S,3R,4R,5S)-5-[(2S,3S,4S,5S)-2,3-epoxy-5-hydroxy-4-methylhexyl]tetrahydro-3,4-dihydroxy-β-methyl-2H-pyran-2-crotonic acid, ester with 9-hydroxynonanoic acid, calcium salt (2:1), dihydrate.
The molecular formula of mupirocin calcium is (C26H43O9)2Ca•2H2O, and the molecular weight is 1075.3. The molecular weight of mupirocin free acid is 500.6. The structural formula of mupirocin calcium is:
Each gram of BACTROBAN cream contains 20 mg (2% w/w) of mupirocin equivalent to 21.5 mg (2.15% w/w) of mupirocin calcium. The inactive ingredients are benzyl alcohol, cetomacrogol 1000, cetyl alcohol, mineral oil, phenoxyethanol, purified water, stearyl alcohol, and xanthan gum.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mupirocin is an RNA synthetase inhibitor antibacterial [see Microbiology (12.4)].
12.3 Pharmacokinetics
Absorption
Systemic absorption of mupirocin through intact human skin is minimal. The systemic absorption of mupirocin was studied following application of BACTROBAN cream 3 times daily for 5 days to various skin lesions greater than 10 cm in length or 100 cm2 in area in 16 adults (aged 29 to 60 years) and 10 children (aged 3 to 12 years). Some systemic absorption was observed as evidenced by the detection of the metabolite, monic acid, in urine. Data from this trial indicated more frequent occurrence of percutaneous absorption in children (90% of subjects) compared with adults (44% of subjects); however, the observed urinary concentrations in children (0.07 to 1.3 mcg per mL [1 pediatric subject had no detectable level]) are within the observed range (0.08 to 10.03 mcg per mL [9 adults had no detectable level]) in the adult population. In general, the degree of percutaneous absorption following multiple dosing appears to be minimal in adults and children.
The effect of the concurrent application of BACTROBAN cream with other topical products has not been studied [see Dosage and Administration (2)].
Elimination
In a trial conducted in 7 healthy adult male subjects, the elimination half-life after intravenous administration of mupirocin was 20 to 40 minutes for mupirocin and 30 to 80 minutes for monic acid.
Metabolism: Following intravenous or oral administration, mupirocin is rapidly metabolized. The principal metabolite, monic acid, demonstrates no antibacterial activity.
Excretion: Monic acid is predominantly eliminated by renal excretion.
12.4 Microbiology
Mupirocin is an RNA synthetase inhibitor antibacterial produced by fermentation using the organism Pseudomonas fluorescens.
Mechanism of Action
Mupirocin inhibits bacterial protein synthesis by reversibly and specifically binding to bacterial isoleucyl-transfer RNA (tRNA) synthetase.
Mupirocin is bactericidal at concentrations achieved by topical administration. Mupirocin is highly protein bound (greater than 97%) and the effect of wound secretions on the minimum inhibitory concentrations (MICs) of mupirocin has not been determined.
Resistance
When mupirocin resistance occurs, it results from the production of a modified isoleucyl-tRNA synthetase, or the acquisition of, by genetic transfer, a plasmid mediating a new isoleucyl-tRNA synthetase. High-level plasmid-mediated resistance (MIC ≥512 mcg/mL) has been reported in increasing numbers of isolates of S. aureus and with higher frequency in coagulase-negative staphylococci. Mupirocin resistance occurs with greater frequency in methicillin-resistant than methicillin-susceptible staphylococci.
Cross Resistance
Due to its mode of action, mupirocin does not demonstrate cross resistance with other classes of antimicrobial agents.
Antimicrobial Activity
Mupirocin has been shown to be active against susceptible isolates of S. aureus and S. pyogenes, both in vitro and in clinical trials [see Indications and Usage (1)]. The following in vitro data are available, but their clinical significance is unknown. Mupirocin is active against most isolates of Staphylococcus epidermidis.
Susceptibility Test Methods
High-level mupirocin resistance (≥512 mcg/mL) may be determined using standard disk diffusion or broth microdilution tests.1,2 Because of the occurrence of mupirocin resistance in methicillin-resistant S. aureus (MRSA), it is appropriate to test MRSA populations for mupirocin susceptibility prior to the use of mupirocin using a standardized method.3,4,5
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long‑term studies in animals to evaluate carcinogenic potential of mupirocin calcium have not been conducted.
Results of the following studies performed with mupirocin calcium or mupirocin sodium in vitro and in vivo did not indicate a potential for genotoxicity: rat primary hepatocyte unscheduled DNA synthesis, sediment analysis for DNA strand breaks, Salmonella reversion test (Ames), Escherichia coli mutation assay, metaphase analysis of human lymphocytes, mouse lymphoma assay, and bone marrow micronuclei assay in mice.
In a fertility/reproductive performance study (with dosing through lactation), mupirocin administered subcutaneously to male and female rats at doses up to 100 mg per kg per day which is 14 times the human topical dose (approximately 60 mg mupirocin per day) based on calculations of dose divided by the entire body surface area, did not result in impaired fertility or impaired reproductive performance attributable to mupirocin.
14 CLINICAL STUDIES
The efficacy of topical BACTROBAN cream for the treatment of secondarily infected traumatic skin lesions (e.g., lacerations, sutured wounds, and abrasions not more than 10 cm in length or 100 cm2 in total area) was compared with that of oral cephalexin in 2 randomized, double‑blind, double‑dummy clinical trials. Clinical efficacy rates at follow‑up in the per-protocol populations (adults and pediatric subjects included) were 96.1% for BACTROBAN cream (n = 231) and 93.1% for oral cephalexin (n = 219). Pathogen eradication rates at follow‑up in the per-protocol populations were 100% for both BACTROBAN cream and oral cephalexin.
Pediatrics
There were 93 pediatric subjects aged 2 weeks to 16 years enrolled per protocol in the secondarily infected skin lesion trials, although only 3 were younger than 2 years of age in the population treated with BACTROBAN cream. Subjects were randomized to either 10 days of topical BACTROBAN cream 3 times daily or 10 days of oral cephalexin (250 mg 4 times daily for subjects greater than 40 kg or 25 mg per kg per day oral suspension in 4 divided doses for subjects less than or equal to 40 kg). Clinical efficacy at follow‑up (7 to 12 days post-therapy) in the per-protocol populations was 97.7% (43 of 44) for BACTROBAN cream and 93.9% (46 of 49) for cephalexin.
15 REFERENCES
- 1.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-sixth Informational Supplement. CLSI document M100-S26. Clinical and Laboratory Standards Institute, 950 West Valley Rd., Suite 2500, Wayne, PA 19087, USA, 2016.
- 2.
- Patel J, Gorwitz RJ, et al. Mupirocin Resistance. Clinical Infectious Diseases. 2009; 49(6): 935-41.
- 3.
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard – Tenth Edition. CLSI document M07-A10. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2015.
- 4.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Diffusion Susceptibility Tests; Approved Standard – Twelfth Edition. CLSI document M02-A12. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2015.
- 5.
- Finlay JE, Miller LA, Poupard JA. Interpretive criteria for testing susceptibility of staphylococci to mupirocin. Antimicrob Agents Chemother. 1997; 41(5):1137-1139.
16 HOW SUPPLIED/STORAGE AND HANDLING
BACTROBAN cream is a white cream that contains 20 mg (2% w/w) of mupirocin per gram in an oil- and water-based emulsion.
BACTROBAN cream, 2% is supplied in 15‑gram and 30‑gram tubes.
NDC 0029-1527-22 (15-gram tube)
NDC 0029-1527-25 (30-gram tube)
Store at or below 25°C (77°F). Do not freeze.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Advise the patient to administer BACTROBAN cream as follows:
- •
- Use BACTROBAN cream only as directed by the healthcare provider. It is for external use only. Avoid contact of BACTROBAN cream with the eyes. If BACTROBAN cream gets in the eyes, rinse thoroughly with water.
- •
- Do not use BACTROBAN cream in the nose.
- •
- Wash your hands before and after applying BACTROBAN cream.
- •
- Use a gauze pad or cotton swab to apply a small amount of BACTROBAN cream to the affected area. The treated area may be covered by gauze dressing if desired.
- •
- Report to the healthcare provider any signs of local adverse reactions. BACTROBAN cream should be stopped and the healthcare provider contacted if irritation, severe itching, or rash occurs.
- •
- Report to the healthcare provider or go to the nearest emergency room if severe allergic reactions, such as swelling of the lips, face, or tongue, or wheezing occur [see Warnings and Precautions (5.1)].
- •
- If no improvement is seen in 3 to 5 days, contact the healthcare provider.
Trademarks are owned by or licensed to the GSK group of companies.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2020 GSK group of companies or its licensor.
BBC:5PI
PHARMACIST‑DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT
|
PATIENT INFORMATION BACTROBAN (BACK-troh-ban) (mupirocin) cream, for topical use |
|
What is BACTROBAN cream? BACTROBAN cream is a prescription medicine used on the skin (topical use) to treat certain skin infections caused by bacteria called Staphylococcus aureus and Streptococcus pyogenes. It is not known if BACTROBAN cream is safe and effective in children under 3 months of age. |
|
Who should not use BACTROBAN cream? Do not use BACTROBAN cream if:
|
|
What should I tell my healthcare provider before using BACTROBAN cream? Before using BACTROBAN cream, tell your healthcare provider about all of your medical conditions including if you:
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Do not mix BACTROBAN cream with other lotions, creams, or ointments. |
|
How should I use BACTROBAN cream?
|
|
What are the possible side effects of BACTROBAN cream? BACTROBAN cream may cause serious side effects, including:
○ hives ○ trouble breathing or wheezing ○ swelling of your face, lips, mouth, or tongue ○ dizziness, fast heartbeat, or pounding in your chest ○ a rash over your whole body
The most common side effects of BACTROBAN cream include:
These are not all the possible side effects of BACTROBAN cream. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store BACTROBAN cream?
|
|
General information about the safe and effective use of BACTROBAN cream. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use BACTROBAN cream for a condition for which it was not prescribed. Do not give BACTROBAN cream to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about BACTROBAN cream that is written for health professionals. |
|
What are the ingredients in BACTROBAN cream? Active Ingredient: mupirocin calcium Inactive Ingredients: benzyl alcohol, cetomacrogol 1000, cetyl alcohol, mineral oil, phenoxyethanol, purified water, stearyl alcohol, and xanthan gum Trademarks are owned by or licensed to the GSK group of companies. ©2020 GSK group of companies or its licensor. |
|
GlaxoSmithKline, Research Triangle Park, NC 27709 BBC:3PIL For more information, call 1-888-825-5249. |
- This Patient Information has been approved by the U.S. Food and Drug Administration Revised: February 2020
| BACTROBAN
mupirocin calcium cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - GlaxoSmithKline LLC (167380711) |