SCRUB CARE- chlorhexidine gluconate solution
Productos Urologos de Mexico, S.A. de C.V.
----------
Scrub Care® 4% CHG Surgical Scrub Brush-Sponge
Use
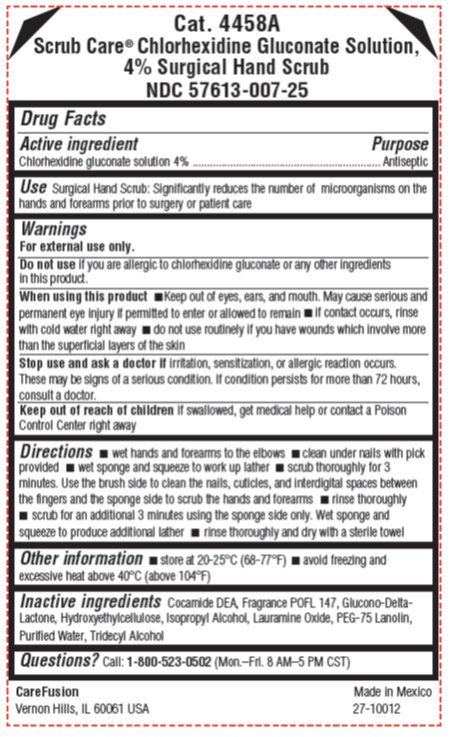
Surgical Hand Scrub: Significantly reduces the number of microorganisms on the hands and forearms prior to surgery or patient care
Warnings
For external use only.
Do not use
if you are allergic to chlorhexidine gluconate or any other ingredients in this product.
When using this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if permitted to enter or allowed to remain
- if contact occurs, rinse with cold water right away
- do not use routinely if you have wounds which involve more than the superficial layers of the skin
Stop use and ask a doctor if
irritation, sensitization, or allergic reaction occurs. These may be signs of a serious condition. If condition persists for more than 72 hours, consult a doctor.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Directions
- wet hands and forearms to the elbows
- clean under nails with pick provided
- wet sponge and squeeze to work up lather
- scrub thoroughly for 3 minutes. Use the brush side to clean the nails, cuticles, and interdigital spaces between the fingers and the sponge side to scrub the hands and forearms.
- rinse thoroughly
- scrub for an additional 3 minutes using the sponge side only. Wet sponge and squeeze to produce additional lather.
- rinse thoroughly and dry with a sterile towel
Other information
- store at 20-25°C (68-77°F)
- avoid freezing and excessive heat above 40°C (above 104°F)
| SCRUB CARE
chlorhexidine gluconate solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Productos Urologos de Mexico, S.A. de C.V. (812552219) |
| Registrant - CareFusion 2200, Inc (832696038) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Productos Urologos de Mexico, S.A. de C.V. | 812552219 | label(57613-007) , pack(57613-007) | |
Revised: 11/2016
Document Id: 40428526-bb82-4368-e054-00144ff8d46c
Set id: 905651a9-00a1-4494-8f0d-7eb322f95458
Version: 6
Effective Time: 20161101
Productos Urologos de Mexico, S.A. de C.V.