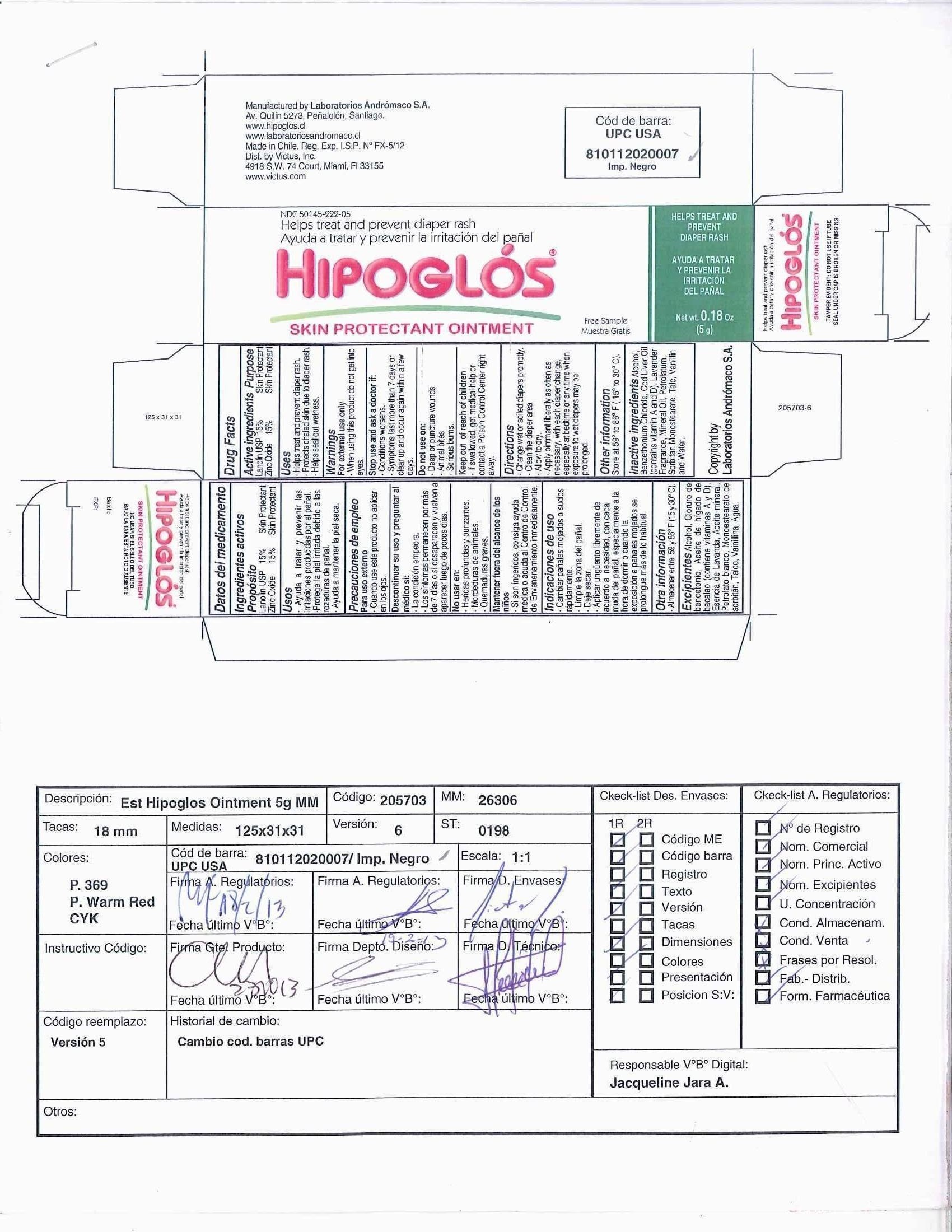
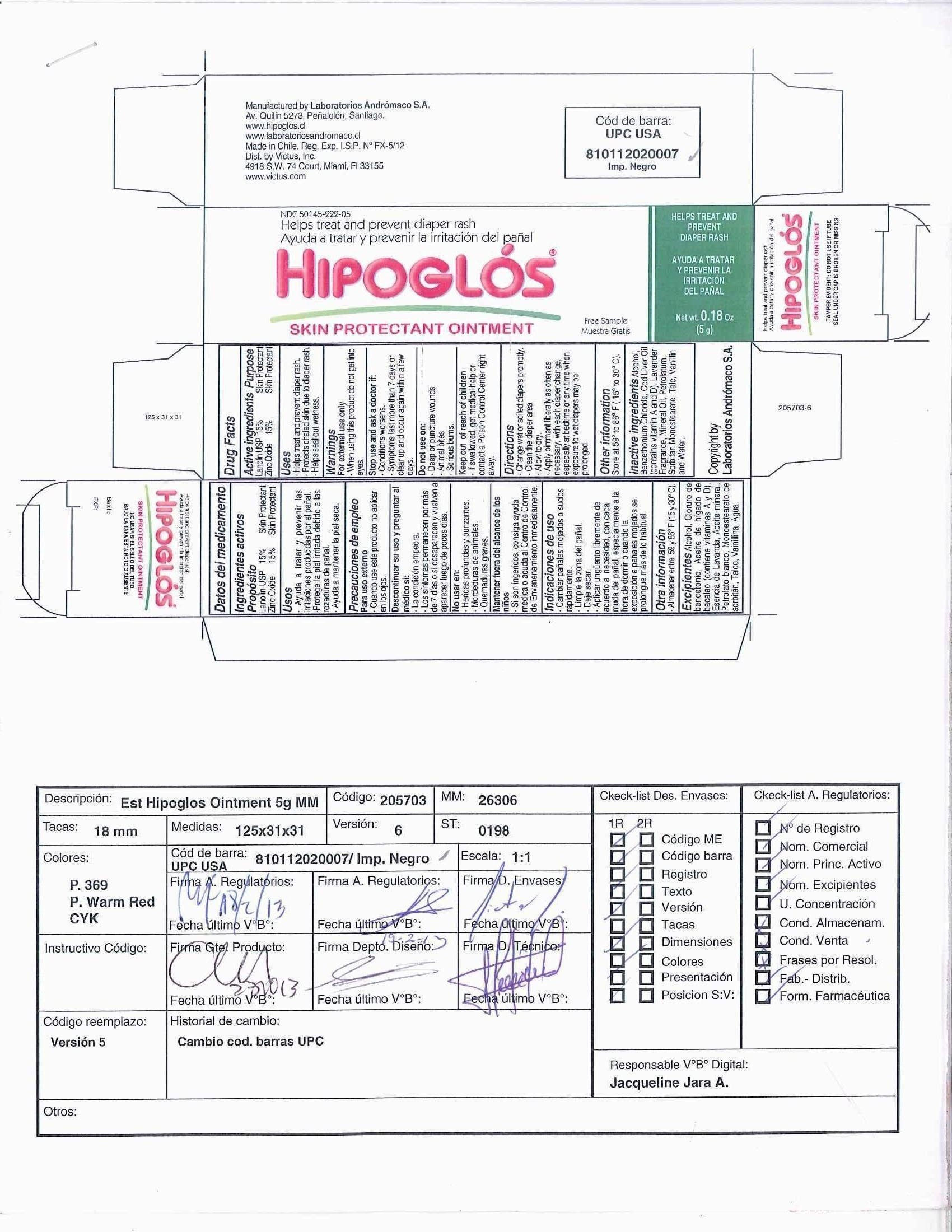
Label: HIPOGLOS- zinc oxide and lanolin ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 50145-222-01, 50145-222-02, 50145-222-03, 50145-222-04, view more50145-222-05 - Packager: Laboratorios Andromaco S.A.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 2, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Keep out of reach of children
- Directions
- Other Information
- Inactive ingredients
- Propósito
- Usos
-
Warnings
Para uso externo
*Cuando use este producto no aplicar en los ojos.Descontinuar su uso y preguntar al médico si:
• la condición empeora
• Los síntomas permanecen por más de 7 días o si desaparecen y vuelven a aparecer luego de pocos días.No usar en:
• Heridas profundas y punzantes.
• Mordeduras de animales
• Quemaduras graves - Mantener fuera del alcance de los niños
- Indicaciones de uso
- Otra información
- Excipientes
- Principal Display Panel - Carton Label
- Principal Display Panel - Tube Label
-
INGREDIENTS AND APPEARANCE
HIPOGLOS
zinc oxide and lanolin ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50145-222 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 15 g in 100 g LANOLIN (UNII: 7EV65EAW6H) (LANOLIN - UNII:7EV65EAW6H) LANOLIN 15 g in 100 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) BENZETHONIUM CHLORIDE (UNII: PH41D05744) COD LIVER OIL (UNII: BBL281NWFG) ENGLISH LAVENDER OIL (UNII: ZBP1YXW0H8) MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) TALC (UNII: 7SEV7J4R1U) VANILLIN (UNII: CHI530446X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50145-222-01 1 in 1 CARTON 1 120 g in 1 TUBE 2 NDC:50145-222-02 1 in 1 CARTON 2 100 g in 1 TUBE 3 NDC:50145-222-03 1 in 1 CARTON 3 72 g in 1 TUBE 4 NDC:50145-222-04 1 in 1 CARTON 4 60 g in 1 TUBE 5 NDC:50145-222-05 1 in 1 CARTON 5 5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 11/03/2010 Labeler - Laboratorios Andromaco S.A. (980106538) Registrant - Laboratorios Andromaco S.A. (980106538) Establishment Name Address ID/FEI Business Operations Laboratorios Andromaco S.A. 980106538 manufacture(50145-222)