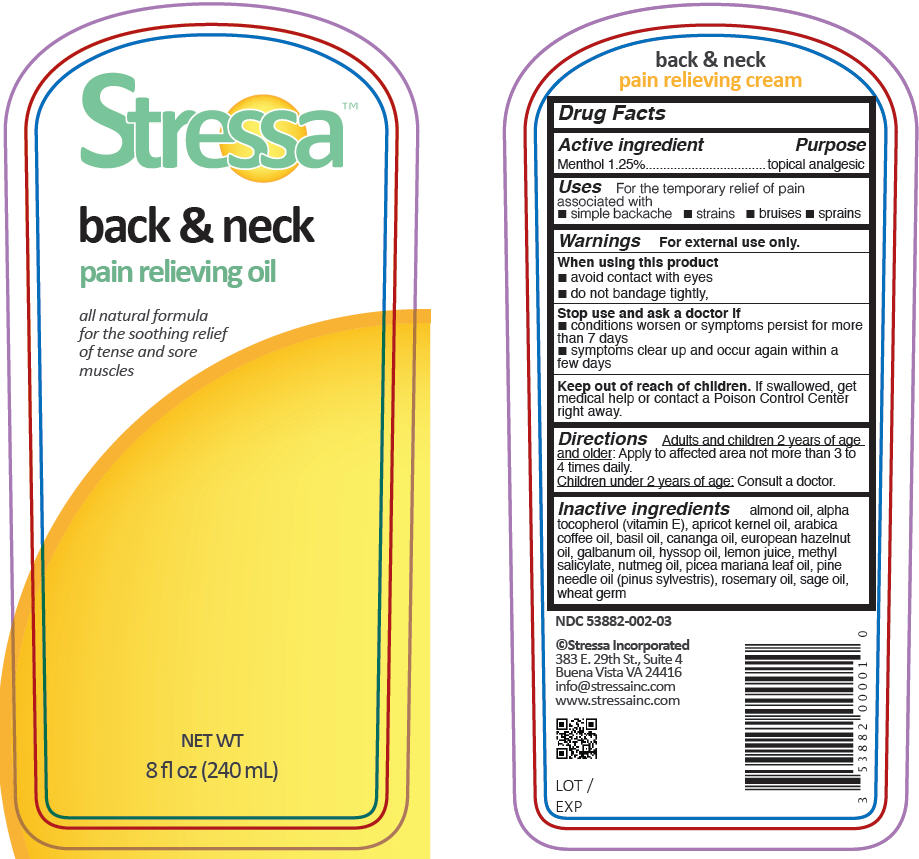
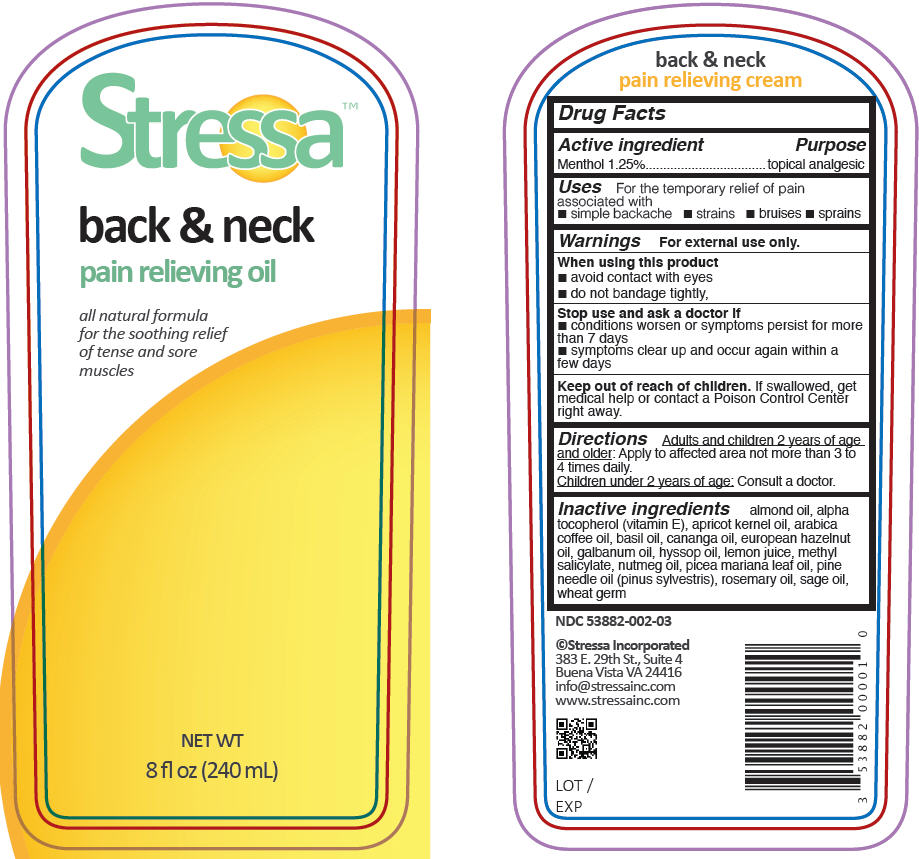
Label: STRESSA BACK AND NECK PAIN RELIEVING- menthol oil
-
Contains inactivated NDC Code(s)
NDC Code(s): 53882-002-01, 53882-002-02, 53882-002-03 - Packager: Stressa Incorporated
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 4, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL - 240 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
STRESSA BACK AND NECK PAIN RELIEVING
menthol oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53882-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Menthol (UNII: L7T10EIP3A) (Menthol - UNII:L7T10EIP3A) Menthol 1 g in 93 mL Inactive Ingredients Ingredient Name Strength ALMOND OIL (UNII: 66YXD4DKO9) APRICOT KERNEL OIL (UNII: 54JB35T06A) BASIL OIL (UNII: Z129UMU8LE) ARABICA COFFEE OIL (UNII: IK55HKE887) GALBANUM OIL (UNII: 211UF7M8N1) EUROPEAN HAZELNUT OIL (UNII: 8RQ2839AVG) HYSSOP OIL (UNII: 173D71924B) LEMON OIL (UNII: I9GRO824LL) NUTMEG OIL (UNII: Z1CLM48948) ROSEMARY OIL (UNII: 8LGU7VM393) SAGE OIL (UNII: U27K0H1H2O) PINE NEEDLE OIL (PINUS SYLVESTRIS) (UNII: 5EXL5H740Y) PICEA MARIANA LEAF OIL (UNII: Q1J49L1A5O) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) WHEAT GERM (UNII: YR3G369F5A) METHYL SALICYLATE (UNII: LAV5U5022Y) CANANGA OIL (UNII: 8YOY78GNNX) Product Characteristics Color ORANGE (Clear) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53882-002-01 60 mL in 1 BOTTLE, PLASTIC 2 NDC:53882-002-02 118 mL in 1 BOTTLE, PLASTIC 3 NDC:53882-002-03 240 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part348 08/01/2014 Labeler - Stressa Incorporated (031377428) Establishment Name Address ID/FEI Business Operations Stressa Incorporated 031377428 MANUFACTURE(53882-002)