CHILDRENS ALLERGY- diphenhydramine hydrochloride liquid
Rite Aid Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
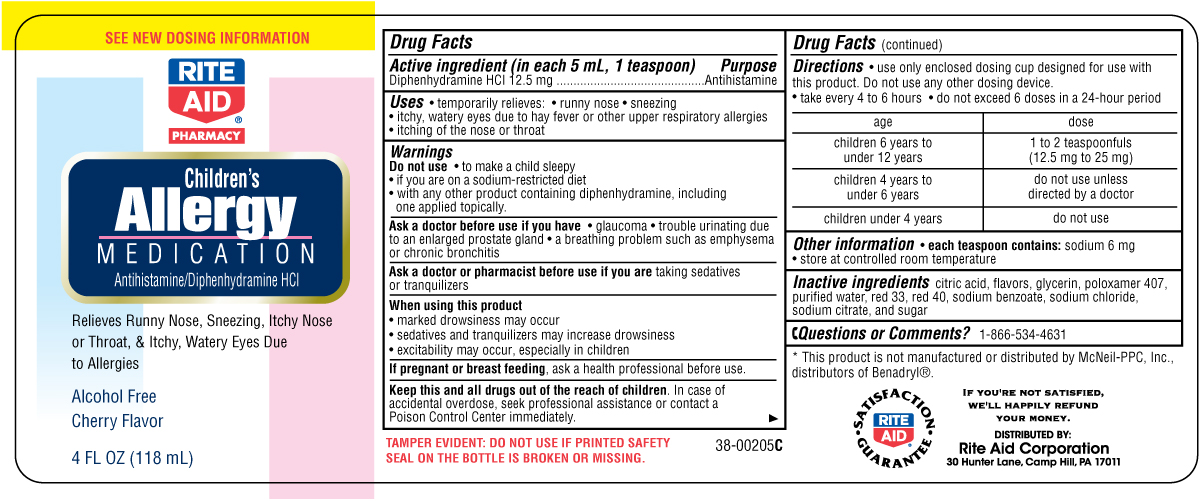
Drug Facts
Uses
• temporarily relieves:
• runny nose • sneezing
• itchy, watery eyes due to hay fever or other
upper respiratory allergies
• itching of the nose or throat
Do Not Use
• to make a child sleepy
• if you are on a sodium-restricted diet
• with any other product containing diphenhydramine,
including one applied topically.
Ask a doctor before use if you have
• glaucoma
• trouble urinating due to an enlarged prostate gland
• a breathing problem such as emphysema or
chronic bronchitis
When using this product
• marked drowsiness may occur
• sedatives and tranquilizers may increase
drowsiness
• excitability may occur, especially in children
Keep this and all drugs out of the reach of children.
In case of accidental overdose, seek professional
assistance or contact a Poison Control Center
immediately.
Directions
• use only enclosed dosing cup designed for use with
this product. Do not use any other dosing device.
• take every 4 to 6 hours
• do not exceed 6 doses in a 24-hour period
age dose
children 6 years to under 12 years 1 to 2 teaspoonfuls (12.5 mg to 25 mg)
children 4 years to under 6 years do not use unless directed by a doctor
children under 4 years do not use
| CHILDRENS ALLERGY
diphenhydramine hydrochloride liquid |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Rite Aid Corporation (014578892) |