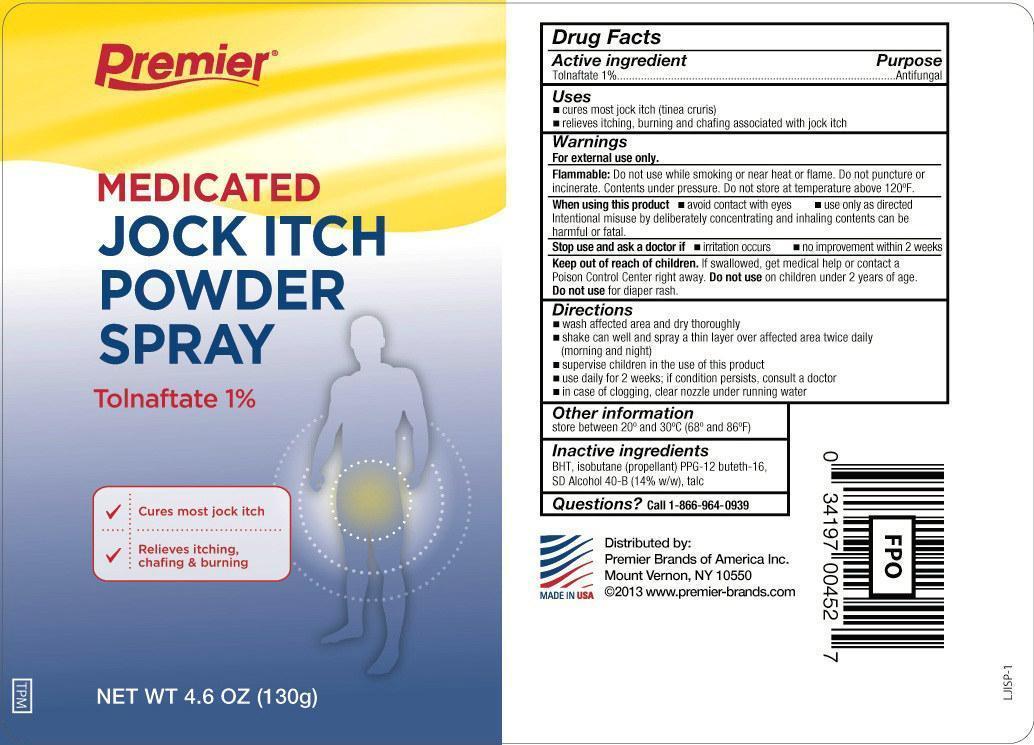
TOLNAFTATE- medicated jock itch powder spray aerosol, powder
Premier Brands of America Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Premier Brands Tolnaftate Jock Itch Powder Spray
Uses
- cures most jock itch (tinea cruris)
- relieves itching, burning and chafing associated with jock itch
Warnings
For external use only.
Flammable:
Do not use while smoking or near heat or flame. Do not puncture or incinerate. Contents under pressure. Do not store at temperature above 120ºF.
Directions
- wash affected area and dry thoroughly
- shake can well and spray a thin layer over affected area twice daily (morning and night)
- supervise children in the use of this product
- use daily for 2 weeks; if conditions persist, consult a doctor
- in case of clogging, clear nozzle under running water
| TOLNAFTATE
medicated jock itch powder spray aerosol, powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Premier Brands of America Inc. (080051232) |
Revised: 1/2021
Document Id: b9be5bd7-5c9b-be91-e053-2995a90a082a
Set id: 8ca068db-07a0-4bd1-96dd-32e858473460
Version: 4
Effective Time: 20210125
Premier Brands of America Inc.