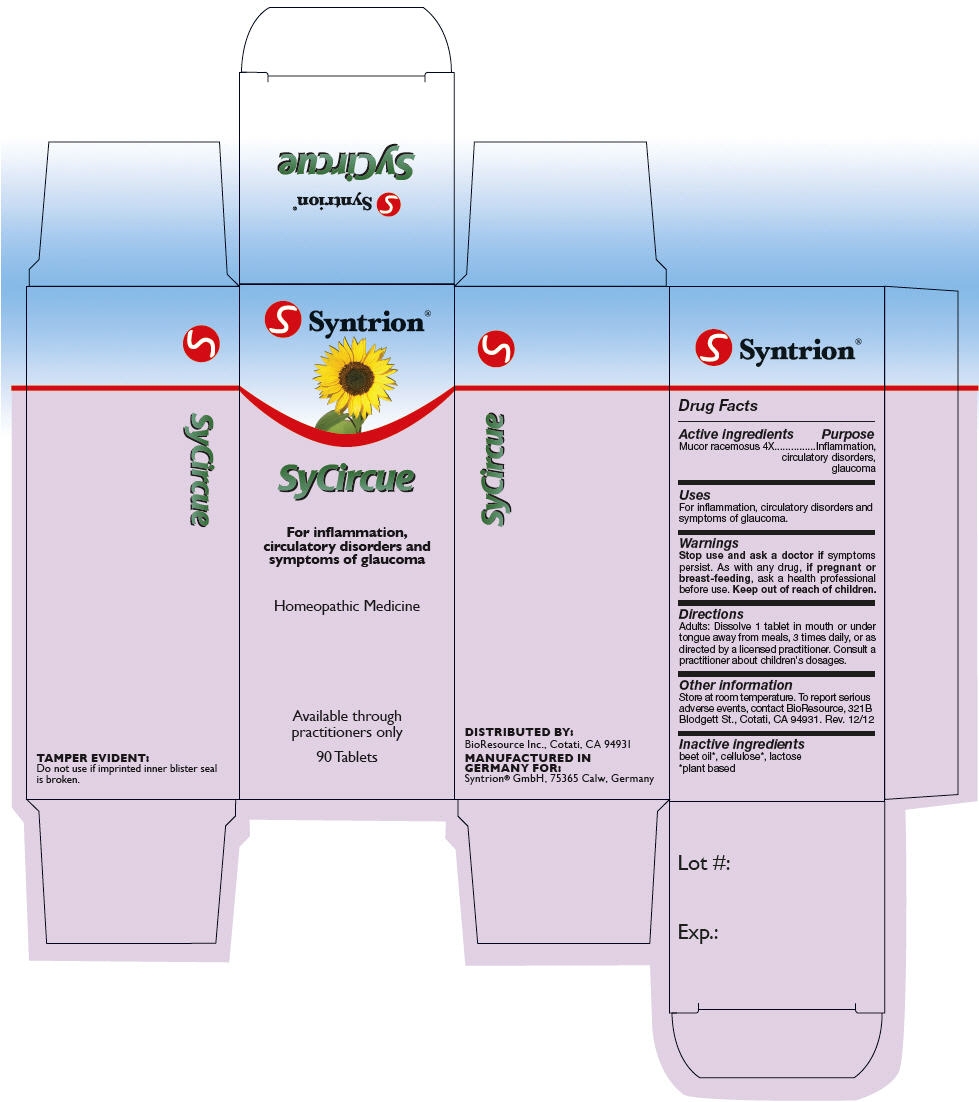
SYCIRCUE- mucor racemosus tablet
Syntrion GmbH
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active ingredients
Mucor racemosus 4X
Purpose
Inflammation, circulatory disorders, glaucoma
Uses
For inflammation, circulatory disorders and symptoms of glaucoma.
Warnings
Stop use and ask a doctor if symptoms persist.
As with any drug, if pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
Directions
Adults
Dissolve 1 tablet in mouth or under tongue away from meals, 3 times daily, or as directed by a licensed practitioner. Consult a practitioner about children's dosages.
Other information
Store at room temperature. To report serious adverse events, contact BioResource, 321B Blodgett St., Cotati, CA 94931. Rev. 12/12
Inactive ingredients
beet oil1, cellulose1, lactose
DISTRIBUTED BY:
BioResource Inc., Cotati, CA 94931
PRINCIPAL DISPLAY PANEL - 90 Tablet Carton
Syntrion®
SyCircue
For inflammation,
circulatory disorders and
symptoms of glaucoma
Homeopathic Medicine
Available through
practitioners only
90 Tablets