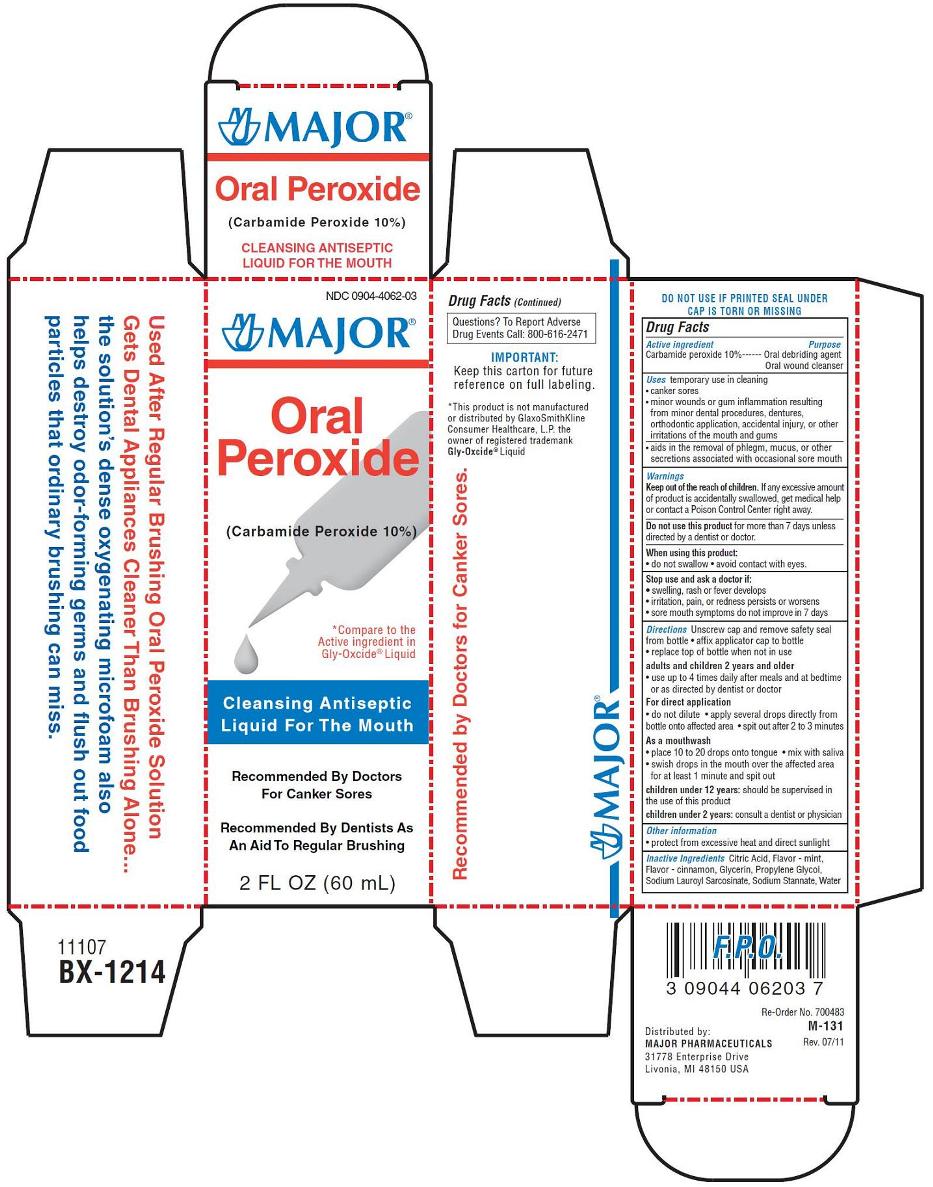
ORAL PEROXIDE- carbamide peroxide liquid
Major Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
- •
- temporary use in cleansing:
- •
- canker sore
- •
- minor wounds or gum inflammation resulting from minor dental procedures, dentures, orthodontic appliances, accidental injury, or other irritations of the mouth and gums
- •
- aids in the removal of phlegm, mucus, or other secretions associated with occasional sore mouth
Warnings
Directions
- •
- unscrew cap and remove safety seal from bottle
- •
- affix applicator cap to bottle
- •
- replace top of bottle when not in use
Adults and children 2 years and older:
- •
- use up to 4 times daily after meals and at bedtime or as directed by dentist or doctor
For direct application
- •
- do not dilute
- •
- apply several drops directly from bottle onto affected area
- •
- spit out after 2 to 3 minutes
As a mouthwash (oral rinse)
- •
- place 10 to 20 drops onto tongue
- •
- mix with saliva
- •
- swish around in the mouth over the affected area for at least 1 minute and spit out
Children under 12 years: should be supervised in the use of this product
Children under 2 years: consult a dentist or doctor
Other information
- •
- protect from excessive heat and direct sunlight
- •
- store bottle in the outer carton
Inactive ingredients
citric acid, flavor - mint, flavor - cinnamon, glycerin, propylene glycol, sodium lauroyl sarcosinate, sodium stannate, water
| ORAL PEROXIDE
carbamide peroxide liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Major Pharmaceuticals (191427277) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Major Pharmaceuticals | 191427277 | REPACK(0904-4062) | |
Revised: 8/2018
Document Id: 35e15407-404c-43a2-b223-bbefd85a5d94
Set id: 8c18a5b0-da4a-4722-a9b8-17bf027debdc
Version: 3
Effective Time: 20180806
Major Pharmaceuticals