NEOSPORIN ORIGINAL- bacitracin, neomycin, polymyxin b
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
NEOSPORIN ORIGINAL OINTMENT
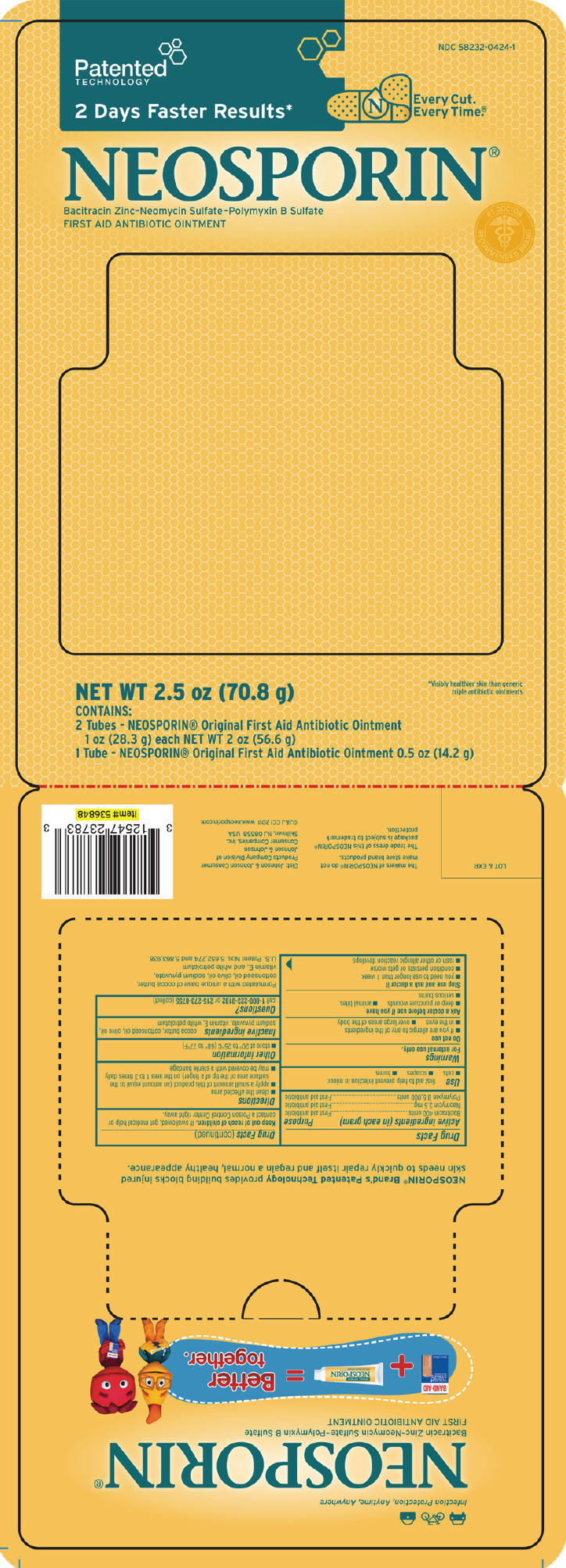
Warnings
For external use only.
Directions
- clean the affected area
- apply a small amount of this product (an amount equal
to the surface area of the tip of a finger) on the area
1 to 3 times daily - may be covered with a sterile bandage
Inactive ingredients
cocoa butter, cottonseed oil, olive oil, sodium pyruvate, vitamin E, white petrolatum
Dist: Johnson & Johnson Consumer
Products Company Division of
Johnson & Johnson
Consumer Companies, Inc.
Skillman, NJ 08558 USA
PRINCIPAL DISPLAY PANEL - 70.8 g Kit
NDC# 58232-0424-1
Patented
TECHNOLOGY
| 2 Days Faster Results* | Every Cut. Every Time® |
| NEOSPORIN®
Bacitracin Zinc-Neomycin Sulfate-Polymyxin B Sulfate FIRST AID ANTIBIOTIC OINTMENT |
|
| NET WT 2.5 OZ (70.8 g) | *Visibly healthier skin than generic triple antibiotic ointments |
| CONTAINS: 2 Tubes - NEOSPORIN® Original First Aid Antibiotic Ointment 1 oz (28.3 g) each NET WT 2 oz (56.6 g) 1 Tube - NEOSPORIN® Original First Aid Antibiotic Ointment 0.5 oz (14.2 g) |
|
| NEOSPORIN ORIGINAL
bacitracin, neomycin, polymyxin b kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |
Revised: 9/2019
Document Id: cd430556-cea5-4878-a307-b5eb135e6335
Set id: 8aeaaddd-16f5-4b98-9626-f60aa2c37c5e
Version: 2
Effective Time: 20190912
Johnson & Johnson Consumer Inc.