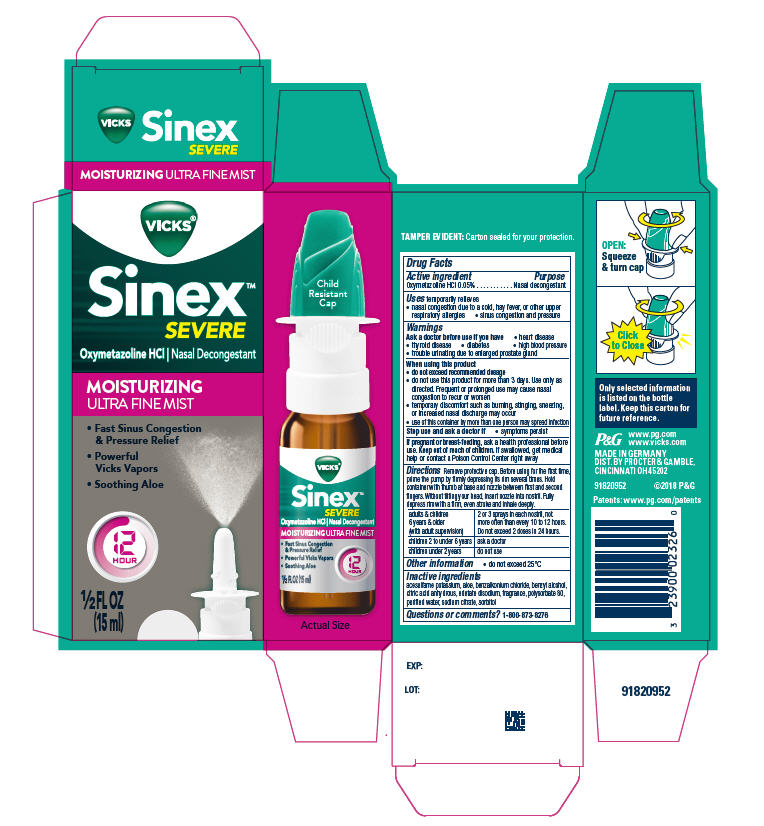
VICKS SINEX 12 HOUR NASAL DECONGESTANT ULTRA FINE MIST MOISTURIZING- oxymetazoline hydrochloride spray
The Procter & Gamble Manufacturing Company
----------
Vicks
®Sinex™ SEVERE
MOISTURIZING
ULTRA FINE MIST
Uses
temporarily relieves
- nasal congestion due to a cold, hay fever, or other upper respiratory allergies
- sinus congestion and pressure
When using this product
- do not exceed recommended dosage
- do not use this product for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen
- temporary discomfort such as burning, stinging, sneezing, or increased nasal discharge may occur
- use of this container by more than one person may spread infection
Directions
Remove protective cap. Before using for the first time, prime the pump by firmly depressing its rim several times. Hold container with thumb at base and nozzle between first and second fingers. Without tilting your head, insert nozzle into nostril. Fully depress rim with a firm, even stroke and inhale deeply.
| adults & children 6 yrs. & older (with adult supervision) | 2 or 3 sprays in each nostril, not more often than every 10 to 12 hours. Do not exceed 2 doses in 24 hours. |
| children 2 to under 6 yrs. | ask a doctor |
| children under 2 yrs. | do not use |
Inactive ingredients
acesulfame potassium, aloe, benzalkonium chloride, benzyl alcohol, citric acid anhydrous, edetate disodium, fragrance, polysorbate 80, purified water, sodium citrate, sorbitol
| VICKS SINEX
12 HOUR NASAL DECONGESTANT ULTRA FINE MIST MOISTURIZING
oxymetazoline hydrochloride spray |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |
Revised: 11/2023
Document Id: 0ab0bbef-fa89-1caa-e063-6294a90a27c8
Set id: 8aa95112-c538-4a55-ab40-ad164d752dd4
Version: 14
Effective Time: 20231121
The Procter & Gamble Manufacturing Company