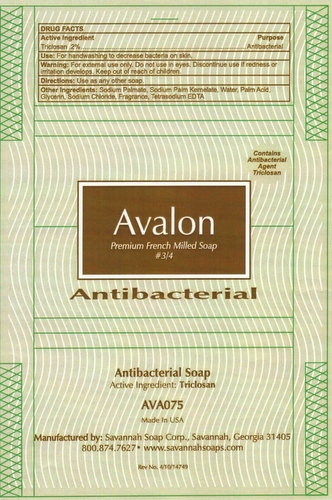
AVALON PREMIUM FRENCH MILLED- triclosan soap
BNP Industries Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Avalon Premium French Milled Soap
Warning: For external use only. Do not use in eyes. Discontinue use if redness or irritation develops. Keep out of reach of children
Other Ingredients: Sodium Palmate, Sodium Palm Kermelate, Water, Palm Acid, Glycerin, Sodium Chloride,Fragrance, Tetrasodium EDTA
| AVALON PREMIUM FRENCH MILLED
triclosan soap |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - BNP Industries Inc. (606982189) |
| Registrant - BNP Industries Inc. (606982189) |
Revised: 7/2017
Document Id: 5432b594-0351-189e-e054-00144ff88e88
Set id: 89995b01-58a9-4998-8196-3ae8e307c496
Version: 2
Effective Time: 20170713
BNP Industries Inc.