VIVAGLOBIN- human immunoglobulin g solution
CSL Behring LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Vivaglobin safely and effectively. See full prescribing information for Vivaglobin.
Vivaglobin Immune Globulin Subcutaneous (Human) 16% Liquid Initial U.S. Approval: 2006 RECENT MAJOR CHANGESINDICATIONS AND USAGEVivaglobin is an Immune Globulin Subcutaneous (Human) (IGSC), 16% Liquid indicated for the treatment of primary humoral immunodeficiency (PI) (1). DOSAGE AND ADMINISTRATIONFor subcutaneous infusion only. DO NOT INJECT INTO A BLOOD VESSEL. Start patients on treatment with Vivaglobin 1 week after having received Immune Globulin Intravenous (Human) (IGIV) infusions at regular intervals for a period of at least 3 months. Initial Weekly Dose (2.3) The initial weekly dose of Vivaglobin is calculated to achieve a systemic serum IgG exposure (area under the concentration-time curve [AUC]) not inferior to the AUC of the previous IGIV treatment.
Dose Adjustment (2.3, Table 1)
Administration (2.4)
DOSAGE FORMS AND STRENGTHS16% IgG (160 mg/mL) for subcutaneous infusion (3) CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions (observed in ≥5% of study subjects) were local injection-site reactions (swelling, redness, and itching), headache, nausea, rash, asthenia, and gastrointestinal disorder (6.1). To report SUSPECTED ADVERSE REACTIONS, contact the CSL Behring Pharmacovigilance Department at 1-866-915-6958 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 7/2010 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Vivaglobin is an Immune Globulin Subcutaneous (Human) (IGSC), 16% Liquid indicated as replacement therapy for primary humoral immunodeficiency (PI). This includes, but is not limited to, the primary immunodeficiency in common variable immunodeficiency (CVID), X-linked agammaglobulinemia, congenital agammaglobulinemia, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies.
2 DOSAGE AND ADMINISTRATION
For subcutaneous infusion only. DO NOT INJECT INTO A BLOOD VESSEL.
2.1 Self-Administration
Self-administration is appropriate for some patients. If self-administration is planned, the healthcare professional should provide the patient with instructions and training for subcutaneous infusion in the home or other appropriate setting (see Patient Counseling Information [17.1] and the FDA-Approved Patient Labeling).
2.2 Preparation and Handling
Vivaglobin is a colorless to light brown solution. Do not use if the solution is cloudy (turbid) or contains particulates.
- Prior to administration, bring the Vivaglobin vial(s) to room temperature. Then, visually inspect each vial for particulate matter by gently swirling the vial, and check for discoloration by holding it up to the light.
- Check the product expiration date on the vial label. Do not use beyond the expiration date.
- Do not mix Vivaglobin with other products.
- Do not shake the Vivaglobin vial.
- Use aseptic technique when preparing and administering Vivaglobin.
- The Vivaglobin vial is for single-use only. Discard all administration equipment and any unused product immediately after each infusion in accordance with local requirements.
2.3 Dosage
The dose should be individualized based on the patient's clinical response to Vivaglobin therapy and serum immunoglobulin (IgG) trough levels.
Begin treatment with Vivaglobin one week after the patient has received a regularly scheduled Immune Globulin Intravenous (Human) (IGIV) infusion. Prior to receiving treatment with Vivaglobin, patients need to have been receiving IGIV treatment for at least 3 months at dosing intervals of either every 3 weeks or every 4 weeks.
The initial weekly dose of Vivaglobin is established by converting the monthly IGIV dose into a weekly equivalent and increasing it using a dose adjustment factor (see Initial Weekly Dose). The goal is to achieve a systemic serum IgG exposure (area under the concentration-time curve [AUC]) not inferior to the AUC of the previous IGIV treatment (see Pharmacokinetics [12.3]).
Prior to switching treatment from IGIV to Vivaglobin, obtain the patient's serum IgG trough level to guide subsequent dose adjustment (see Dose Adjustment). After 2 to 3 months, weekly administration of Vivaglobin will lead to stable steady-state serum IgG levels with lower IgG peak levels and higher IgG trough levels compared with monthly IGIV treatment.
Initial Weekly Dose
To calculate the initial weekly dose of Vivaglobin, multiply the previous IGIV dose in grams (g) by the dose adjustment factor of 1.37, then divide this dose by the number of weeks between doses during the patient's previous IGIV treatment (i.e., 3 or 4).
| IGSC weekly dose (g) = | 1.37 × previous IGIV dose (g) |
| Number of weeks between IGIV doses |
To convert the Vivaglobin dose (g) to milliliters (mL), divide the dose in grams (g) by 0.16.
Dose Adjustment
Over time, the dose may need to be adjusted to achieve the desired clinical response and serum IgG trough level. To determine if a dose adjustment should be considered, measure the patient's serum IgG trough level on IGIV prior to switching to Vivaglobin and every 2 to 3 months after switching from IGIV to Vivaglobin.
To achieve the same AUC with Vivaglobin as with the previous IGIV treatment, follow these steps:
-
Estimate the target serum IgG trough level on weekly Vivaglobin treatment, which is derived as follows:
Target concentration (mg/dL) during Vivaglobin treatment = the last trough level during prior IGIV treatment + 180 mg/dL -
In Table 1, find the additional Vivaglobin dose to be administered, based on the patient's body weight, and the difference between the target IgG concentration (mg/dL) and the observed trough level during Vivaglobin treatment.
Additional dosage increments may be indicated based on the patient's clinical response (infection frequency and severity).
| Difference From Target IgG Trough Level* (mg/dL) | Body Weight (kg) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 15 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 110 | 120 | |
| Dose Adjustment (mL per Week)† | |||||||||||||
|
|||||||||||||
| 100 | 1 | 2 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 150 | 2 | 2 | 3 | 5 | 6 | 8 | 9 | 11 | 12 | 14 | 15 | 17 | 18 |
| 200 | 2 | 3 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 23 | 25 |
| 250 | 3 | 4 | 5 | 8 | 10 | 13 | 15 | 18 | 20 | 23 | 26 | 28 | 31 |
| 300 | 3 | 5 | 6 | 9 | 12 | 15 | 18 | 22 | 25 | 28 | 31 | 34 | 37 |
| 350 | 4 | 5 | 7 | 11 | 14 | 18 | 22 | 25 | 29 | 32 | 36 | 39 | 43 |
| 400 | 4 | 6 | 8 | 12 | 16 | 20 | 25 | 29 | 33 | 37 | 41 | 45 | 49 |
| 450 | 5 | 7 | 9 | 14 | 18 | 23 | 28 | 32 | 37 | 41 | 46 | 51 | 55 |
| 500 | 5 | 8 | 10 | 15 | 20 | 26 | 31 | 36 | 41 | 46 | 51 | 56 | 61 |
For example, if a patient with a body weight of 70 kg has an actual IgG trough level of 900 mg/dL and the target trough level is 1000 mg/dL, this results in a difference of 100 mg/dL. Therefore, increase the weekly dose of Vivaglobin by 7 mL.
Monitor the patient's clinical response, and repeat the dose adjustment process as needed.
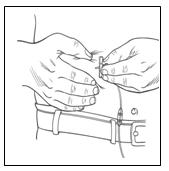
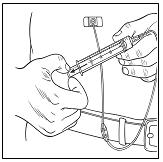
2.4 Administration
Vivaglobin is for subcutaneous infusion only. DO NOT INJECT INTO A BLOOD VESSEL.
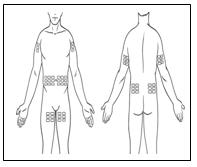
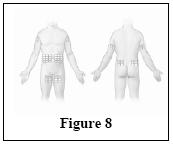
Vivaglobin is for subcutaneous infusion, preferably in the abdomen, thigh, upper arm, and/or lateral hip. Multiple injection sites should be at least two inches apart, and the actual point of injection should be changed with each weekly administration.
- Infusion volume – Do not exceed 15 mL per site. Divide doses greater than 15 mL and infuse into a maximum of three simultaneous sites for children weighing less than 45 kg (99 pounds), a maximum of six simultaneous sites for adults up to age 65, and a maximum of four simultaneous sites for patients 65 years of age and older. If necessary, additional sites can be used consecutively during an infusion.
- Infusion rate – The maximum recommended infusion rate is 20 mL per hour per site and should not exceed a total of 3.0 mg/kg/minute (1.13 mL/kg/hour) for all simultaneous injection sites combined.
Ensure that patients are not volume depleted.
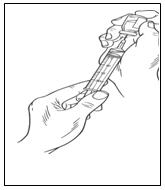
Follow the steps below and use aseptic technique to administer Vivaglobin. For information about subcutaneous infusion in the home or other appropriate setting, see Patient Counseling Information (17.1).
|
|
|
|
|
|
|  |
|
|
|  |
|
|
|  |
|
|
|  |
|
|
|
|
After administration, immediately discard any unused product and administration equipment in accordance with local procedures.
3 DOSAGE FORMS AND STRENGTHS
Vivaglobin is a solution containing 16% IgG (160 mg/mL) for subcutaneous infusion.
4 CONTRAINDICATIONS
Vivaglobin is contraindicated in patients who have had an anaphylactic or severe systemic reaction to the administration of Immune Globulin (Human).
Vivaglobin is contraindicated in IgA-deficient patients with antibodies against IgA or a history of hypersensitivity (see Description [11]).
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Severe hypersensitivity reactions may occur (see Patient Counseling Information [17.2]). In case of hypersensitivity, discontinue the Vivaglobin infusion immediately and institute appropriate treatment. Epinephrine should be immediately available to treat any acute severe hypersensitivity reactions.
Individuals with IgA deficiency can develop anti-IgA antibodies and anaphylactic reactions (including anaphylaxis and shock) after administration of blood components containing IgA. Patients with known antibodies to IgA may have a greater risk of developing potentially severe hypersensitivity and anaphylactic reactions. Vivaglobin contains ≤1.7 mg/mL IgA (see Description [11]). The minimum concentration of IgA that will provoke a hypersensitivity reaction is not known; therefore all IgG preparations carry the risk of inducing an anaphylactic reaction to IgA.
5.2 Aseptic Meningitis Syndrome (AMS)
AMS has been reported to occur infrequently with IGIV treatment5 and with Vivaglobin treatment. The syndrome usually begins within several hours to 2 days following IGIV treatment. AMS is characterized by signs and symptoms including severe headache, nuchal rigidity, drowsiness, fever, photophobia, painful eye movements, nausea, and vomiting. Cerebrospinal fluid (CSF) studies frequently show pleocytosis up to several thousand cells per cubic millimeter, predominantly from the granulocytic series, and with elevated protein levels up to several hundred mg/dL. AMS may occur more frequently in association with high doses (2 g/kg) and/or rapid infusion of IGIV.
Patients exhibiting such signs and symptoms should receive a thorough neurological examination, including CSF studies, to rule out other causes of meningitis. Discontinuation of IGIV treatment has resulted in remission of AMS within several days without sequelae.
5.3 Reactions Reported with IGIV Treatment
The following reactions have been reported to occur with IGIV treatment and may occur with IGSC treatment.
Renal Dysfunction/Failure
Renal dysfunction/failure, osmotic nephropathy, and death may occur with use of human immune globulin products. Ensure that patients are not volume depleted and assess renal function, including measurement of blood urea nitrogen (BUN) and serum creatinine, before the initial infusion of Vivaglobin and at appropriate intervals thereafter.
Periodic monitoring of renal function and urine output is particularly important in patients judged to have a potential increased risk of developing acute renal failure.1 If renal function deteriorates, consider discontinuing Vivaglobin. For patients judged to be at risk of developing renal dysfunction because of pre-existing renal insufficiency or predisposition to acute renal failure (such as those with diabetes mellitus or hypovolemia, those who are overweight or use concomitant nephrotoxic medicinal products, or those who are over 65 years of age), administer Vivaglobin at the minimum rate practicable.
Thrombotic Events
Thrombotic events may occur with use of human immune globulin products.2-4 Patients at risk may include those with a history of atherosclerosis, multiple cardiovascular risk factors, advanced age, impaired cardiac output, hypercoagulable disorders, prolonged periods of immobilization, and/or known or suspected hyperviscosity. Because of the potentially increased risk of thrombosis, consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies. For patients judged to be at risk of developing thrombotic events, administer Vivaglobin at the minimum rate practicable.
Hemolysis
Vivaglobin may contain blood group antibodies that may act as hemolysins and induce in vivo coating of red blood cells (RBCs) with immunoglobulin, causing a positive direct antiglobulin (Coombs') test result and hemolysis.6-8 Delayed hemolytic anemia can develop subsequent to immune globulin therapy due to enhanced RBC sequestration, and acute hemolysis, consistent with intravascular hemolysis, has been reported.9
Monitor recipients of Vivaglobin for clinical signs and symptoms of hemolysis. If these are present after Vivaglobin infusion, perform appropriate confirmatory laboratory testing. If transfusion is indicated for patients who develop hemolysis with clinically compromising anemia after receiving Vivaglobin, perform adequate cross-matching to avoid exacerbating on-going hemolysis.
Transfusion-Related Acute Lung Injury (TRALI)
Noncardiogenic pulmonary edema may occur in patients administered human immune globulin products.10 TRALI is characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever. Typically, it occurs within 1 to 6 hours following transfusion. Patients with TRALI may be managed using oxygen therapy with adequate ventilatory support.
Monitor recipients of Vivaglobin for pulmonary adverse reactions. If TRALI is suspected, perform appropriate tests for the presence of anti-neutrophil antibodies in both the product and patient's serum.
5.4 Transmissible Infectious Agents
Because Vivaglobin is made from human plasma, it may carry a risk of transmitting infectious agents, e.g., viruses and, theoretically, the Creutzfeldt-Jakob (CJD) agent. No cases of transmission of viral diseases or CJD have been associated with the use of Vivaglobin. Report all infections thought possibly to have been transmitted by Vivaglobin to the CSL Behring Pharmacovigilance Department at 1-866-915-6958 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. The physician should discuss the risks and benefits of this product with the patient before prescribing or administering it to the patient (see Patient Counseling Information [17.2]).
5.5 Laboratory Tests
After infusion of IgG, the transitory rise of the various passively transferred antibodies in the patient's blood may yield positive serological testing results, with the potential for misleading interpretation. Passive transmission of antibodies to erythrocyte antigens (e.g., A, B, and D) may cause a positive direct or indirect antiglobulin (Coombs') test.
6 ADVERSE REACTIONS
The most common adverse reactions (those AEs considered by the investigator to be at least possibly related to Vivaglobin administration) observed in ≥5% of study subjects receiving Vivaglobin were local injection-site reactions (swelling, redness, and itching), headache, nausea, rash, asthenia, and gastrointestinal disorder.
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
US-Canada Study
The safety of Vivaglobin was evaluated in a clinical study in the US and Canada for 12 months in 65 subjects with PI who had been previously treated with IGIV every 3 or 4 weeks (see Clinical Studies [14.1]). After 3 months, subjects were switched from IGIV to weekly subcutaneous administration of Vivaglobin for 12 months. Subjects were treated weekly with Vivaglobin at a mean dose of 158 mg/kg body weight (range: 34 to 352 mg/kg). The 65 subjects received a total of 3,656 infusions of Vivaglobin.
Table 2 shows the number of subjects who withdrew from the US-Canada study due to adverse events (AEs) and the AEs leading to discontinuation.
| AEs | Subjects with AEs At Least Possibly Related | Subjects with AEs Irrespective of Causality | Total Number (%) of Subjects |
|---|---|---|---|
|
|||
| Subjects with at least 1 AE leading to discontinuation | 4 | 1 | 5 (8%) |
| Injection-site reaction | 3 | – | 3 (5%) |
| Intestinal obstruction | – | 1 | 1 (2%) |
| Hyperventilation | 1* | – | 1 (2%) |
| Tachycardia | 1* | – | 1 (2%) |
Table 3 summarizes the most frequent AEs (experienced by more than 5% of subjects), irrespective of causality. It includes all AEs and those considered temporally associated with the Vivaglobin infusion, i.e., occurring during the infusion or within 72 hours after the end of the infusion.
| Table 3: Incidence of Subjects With Adverse Events (AEs)* (Experienced by >5% of Subjects) and Rate† per Infusion, Irrespective of Causality, in the US-Canada Study | ||||
|---|---|---|---|---|
| All AEs | AEs Occurring During or Within 72 Hours of Infusion | |||
| AEs*
(>5% of Subjects) | Number (%) of Subjects (n=65) | Number (Rate†) of AEs per Infusion (n=3656) | Number (%) of Subjects (n=65) | Number (Rate†) of AEs per Infusion (n=3656) |
| AEs at the injection site‡ | 60 (92%) | 1789 (0.49) | 60 (92%) | 1767 (0.4848) |
| Other AEs | ||||
| Headache | 31 (48%) | 159 (0.04) | 30 (46%) | 104 (0.033) |
| Gastrointestinal disorder | 24 (37%) | 35 (0.01) | 18 (28%) | 24 (0.007) |
| Fever | 16 (25%) | 28 (0.008) | 12 (8%) | 20 (0.005) |
| Nausea | 12 (18%) | 18 (0.005) | 11 (17%) | 15 (0.004) |
| Rash | 11 (17%) | 22 (0.006) | 10 (15%) | 16 (0.004) |
| Sore throat | 10 (15%) | 17 (0.005) | 8 (12%) | 11 (0.003) |
| Allergic reaction | 7 (11%) | 8 (0.002) | 5 (8%) | 5 (0.001) |
| Pain | 6 (9%) | 8 (0.002) | 4 (6%) | 4 (0.001) |
| Diarrhea | 6 (9%) | 6 (0.002) | 5 (8%) | 5 (0.001) |
| Cough increased | 6 (9%) | 6 (0.002) | 5 (8%) | 5 (0.001) |
| Gastrointestinal pain | 5 (8%) | 6 (0.002) | 4 (6%) | 5 (0.001) |
| Migraine | 5 (8%) | 5 (0.001) | 2 (3%) | 2 (0.001) |
| Skin disorder | 5 (8%) | 7 (0.002) | 3 (5%) | 5 (0.001) |
| Asthma | 5 (8%) | 8 (0.002) | 3 (5%) | 4 (0.001) |
| Arthralgia | 4 (6%) | 4 (0.001) | 3 (5%) | 3 (0.001) |
| Asthenia | 4 (6%) | 4 (0.001) | 2 (3%) | 2 (0.001) |
| Malaise | 4 (6%) | 5 (0.001) | 2 (3%) | 2 (0.001) |
The total number of AEs, irrespective of causality, including injection-site reactions, that began during or within 72 hours after the end of an infusion was 2262 (a rate of 0.62 AEs per infusion); excluding injection-site reactions, the rate of AEs per infusion was 0.14.
Table 4 summarizes the severity of local AEs by infusion, irrespective of causality.
| Table 4: Severity of Local Adverse Events (AEs) by Infusion, Irrespective of Causality, in the US-Canada Study | ||
|---|---|---|
| AEs | Number (Rate*) of AEs | Number (Rate*) of AEs Occurring During or Within 72 Hours of Infusion |
| (Number of infusions: 3656) |
||
| AEs at the injection site | 1789 (0.49) | 1767 (0.48) |
| Mild† | 1112 (0.30) | 1100 (0.30) |
| Moderate‡ | 601 (0.16) | 593 (0.16) |
| Severe§ | 65 (0.02) | 64 (0.02) |
| Unknown severity | 11 (<0.01) | 10 (<0.01) |
| Discontinuations due to AEs at the injection site | 3 subjects | |
Of the three subjects who discontinued the study due to injection-site reactions, one withdrew on Day 1 (Infusion 1) of the wash-in/wash-out period after a moderate injection-site reaction and a mild headache; one withdrew on Day 22 (Infusion 4) of the wash-in/wash-out period following severe injection-site reactions for two weeks; and one withdrew on Day 78 following a mild injection-site reaction.
Local reactions decreased substantially after repeated use.
Table 5 summarizes the most frequent adverse reactions (experienced by at least 3% of subjects) and considered by the investigator to be at least possibly related to Vivaglobin administration.
| Related Adverse Reactions (≥3% Subjects) | Number (%) of Subjects (n=65) | Number (Rate*) of Adverse Reactions per Infusion (n=3656) |
|---|---|---|
| Adverse reactions at the injection site† | 60 (92%) | 1787 (0.49) |
| Other Adverse reactions | ||
| Headache | 21 (32%) | 59 (0.016) |
| Nausea | 7 (11%) | 9 (0.002) |
| Rash | 4 (6%) | 9 (0.002) |
| Asthenia | 3 (5%) | 3 (0.001) |
| Gastrointestinal disorder | 3 (5%) | 3 (0.001) |
| Fever | 2 (3%) | 2 (0.001) |
| Skin disorder | 2 (3%) | 3 (0.001) |
| Tachycardia | 2 (3%) | 2 (0.001) |
| Urine abnormality | 2 (3%) | 3 (0.001) |
Europe-Brazil Study
In a clinical study conducted in Europe and Brazil, the efficacy and safety of Vivaglobin were evaluated for 10 months in 60 subjects with PI. Subjects were treated weekly with Vivaglobin at a mean dose of 89 mg/kg body weight (range: 51 to 147 mg/kg), which was 101% of their previous weekly IGIV or IGSC dose (see Clinical Studies [14.2]). Study subjects received a total of 2,297 infusions of Vivaglobin.
The AEs and their rates reported in this study were similar to those reported in the US-Canada study, with two exceptions: no episodes of headache were reported; and 18 (a rate of 0.008 per infusion) episodes of fever were judged to be related to the administration of Vivaglobin. One subject discontinued due to repeated local reactions of moderate severity.
6.2 Postmarketing Experience
Because postmarketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate the frequency of these reactions or establish a causal relationship to product exposure.
Vivaglobin
Adverse reactions identified during worldwide postmarketing use of Vivaglobin for treatment of PI are allergic-anaphylactic reactions (including dyspnea, pruritus, urticaria, rash, edema and other cutaneous reactions, wheezing, syncope, hypotension, and throat swelling), generalized reactions (including flu-like symptoms, myalgia, chills, fever, tachycardia, arthralgia, nausea and vomiting, diarrhea, gastrointestinal cramping, stomach pain, back pain, headache, headache possibly caused by increased blood pressure, and chest tightness), migraine, and injection-site reactions.
General
The following adverse reactions have been identified and reported during the postmarketing use of IGIV products11:
- Renal: Acute renal dysfunction/failure, osmotic nephropathy
- Respiratory: Apnea, Acute Respiratory Distress Syndrome (ARDS), TRALI, cyanosis, hypoxemia, pulmonary edema, dyspnea, bronchospasm
- Cardiovascular: Cardiac arrest, thromboembolism, vascular collapse, hypotension
- Neurological: Coma, loss of consciousness, seizures, tremor, aseptic meningitis syndrome
- Integumentary: Stevens-Johnson syndrome, epidermolysis, erythema multiforme, bullous dermatitis
- Hematologic: Pancytopenia, leukopenia, hemolysis, positive direct antiglobulin (Coombs') test
- General/Body as a Whole: Pyrexia, rigors
- Musculoskeletal: Back pain
- Gastrointestinal: Hepatic dysfunction, abdominal pain
7 DRUG INTERACTIONS
7.1 Live Virus Vaccines
The passive transfer of antibodies with immunoglobulin administration may interfere with the response to live virus vaccines such as measles/mumps/rubella and varicella (see Patient Counseling Information [17.2]).
8 USE IN SPECIFIC POPULATIONS
8.4 Pediatric Use
- In the US-Canada study, Vivaglobin was evaluated in 6 children (ages 5 through 11) and 4 adolescents (ages 13 through 16). In the Europe-Brazil study, Vivaglobin was evaluated in 16 children (ages 3 through 11) and 6 adolescents (ages 13 through 16).
- The safety and efficacy of Vivaglobin were not studied in pediatric subjects under 2 years of age.
- There were no differences in the safety and efficacy profiles as compared with adult subjects.
- No pediatric-specific dosing requirements were necessary to achieve the desired serum IgG levels.
- For recommendations on the number of simultaneous injection sites for pediatric patients who weigh less than 45 kg (99 pounds), see Administration (2.4).
8.5 Geriatric Use
The clinical studies of Vivaglobin did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger subjects. For recommendations on the number of simultaneous injection sites for geriatric patients, see Administration (2.4).
11 DESCRIPTION
Vivaglobin is a sterile solution consisting of pasteurized, polyvalent immune globulin for subcutaneous administration. Vivaglobin is manufactured from large pools of human plasma by cold alcohol fractionation and is not chemically altered or enzymatically degraded. Vivaglobin is a 16% (160 mg/mL) protein solution, with a content of at least 96% IgG. Vivaglobin contains ≤1.7 mg/mL IgA and ≤1.8 mg/mL IgM. The distribution of IgG subclasses is similar to that present in normal human plasma. Vivaglobin also contains 2.25% glycine, 0.3% sodium chloride, and water for injection, USP. The pH of Vivaglobin is 6.4 to 7.2. Vivaglobin contains no preservative.
All plasma units used in the manufacture of Vivaglobin have been tested using serological assays for hepatitis B surface antigen and antibodies to HIV-1/2 and HCV as well as Nucleic Acid Testing (NAT) for HIV-1 and HCV and found to be nonreactive (negative). For HBV, an investigational NAT procedure is used and the plasma found to be negative; however, the significance of a negative result has not been established. In addition, the plasma has been tested by NAT for HAV and B19V. Only plasma that passed virus screening is used for production, and the limit for B19V in the fractionation pool is set not to exceed 104 IU of B19V DNA per mL.
The capacity of the manufacturing process to remove and/or inactivate enveloped and non-enveloped viruses has been validated by laboratory spiking studies on a scaled-down process model, using enveloped and non-enveloped viruses. The virus reduction capacity of two steps (ethanol – fatty alcohol / pH precipitation and pasteurization in aqueous solution at 60°C for 10 hours) was evaluated. Total mean cumulative virus reductions ranged from 9.0 to ≥14.1 log10 as shown in Table 6.
| Virus Studied | Ethanol – Fatty Alcohol / pH Precipitation [log10] | Pasteurization [log10] | Total Cumulative [log10] |
|---|---|---|---|
| HIV-1, human immunodeficiency virus type 1, model for HIV-1/2; BVDV, bovine viral diarrhea virus, model for HCV and WNV (West Nile virus); PRV, pseudorabies virus, model for large enveloped DNA viruses (e.g., herpes virus); PEV, porcine enterovirus, model for HAV (in an immune globulin product); CPV, canine parvovirus, model for B19V. | |||
|
|||
| Enveloped Viruses | |||
| HIV-1 | ≥6.2 | ≥6.5 | ≥12.7 |
| BVDV | ≥5.3 | ≥8.7 | ≥14.0 |
| WNV | ≥4.4 | ≥9.3 | ≥13.7 |
| PRV | ≥6.2 | ≥7.9 | ≥14.1 |
| Non-enveloped Viruses | |||
| PEV | ≥6.7 | 3.7 | ≥10.4 |
| CPV | 6.7 | 2.3* | 9.0 |
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Vivaglobin supplies a broad spectrum of opsonizing and neutralizing IgG antibodies against a wide variety of bacterial and viral agents. The mechanism of action in PI has not been fully elucidated.
12.3 Pharmacokinetics
The bioavailability of Vivaglobin is approximately 73% compared with IGIV, but can vary significantly among patients (see Clinical Studies [14.1]). With Vivaglobin, peak serum IgG levels are lower than those achieved with IGIV. Subcutaneous administration results in relatively stable steady-state serum IgG levels when the product is dosed on a weekly basis.12-13
The pharmacokinetics (PK) of Vivaglobin were evaluated in the clinical study conducted in the US and Canada (see Clinical Studies [14.1]). Subjects previously treated with IGIV were switched to weekly subcutaneous treatment with Vivaglobin. After a 3-month wash-in/wash-out period, doses were adjusted individually aiming to provide an IgG systemic exposure (AUC) that was not inferior to the AUC of the previous weekly-equivalent IGIV dose.
For the 19 per-protocol subjects completing the wash-in/wash-out period, the average dose adjustment for Vivaglobin was 137% ± 21% SD (range: 103% to 192%) of the previous weekly-equivalent IGIV dose. Following 10 to 12 weeks of treatment with Vivaglobin at this individually adjusted dose, the final steady-state AUC determinations were made in 17 of the 19 per-protocol subjects. The geometric mean ratio of the steady-state AUCs, standardized to a weekly treatment period, for Vivaglobin versus IGIV treatment, was 94.5% (range: 71.4% to 110.1%) with a lower 95% confidence limit of 89.8% for the 17 subjects.
Table 7 summarizes additional pharmacokinetic parameters for this study including dosing and serum IgG peak and trough levels following treatment with IGIV and Vivaglobin.
| IGIV | Vivaglobin | |
|---|---|---|
|
||
| Number of Subjects | 17 | 17 |
| Dose* | ||
| Mean | 120 mg/kg | 165 mg/kg |
| Range | 55-243 mg/kg | 63-319 mg/kg |
| IgG peak levels | ||
| Mean | 1735 mg/dL | 1163 mg/dL |
| Range | 1110-3230 mg/dL | 743-2240 mg/dL |
| IgG trough levels | ||
| Mean | 883 mg/dL | 1064 mg/dL |
| Range | 430-1600 mg/dL | 547-2140 mg/dL |
The 6-month clinical study conducted in Europe and Brazil in 60 subjects with PI also included a PK evaluation. Subjects were treated weekly with Vivaglobin at a mean dose of 89 mg/kg body weight (range: 51 to 147 mg/kg), which was 101% of their previous weekly IGIV or IGSC dose (see Clinical Studies [14.2]). After the subjects had reached steady state with weekly administration of Vivaglobin, peak serum IgG levels were observed after a mean of 2.5 days (range: 0 to 7 days) in 41 subjects.
In both studies, the serum IgG levels in subjects receiving weekly subcutaneous therapy with Vivaglobin were relatively stable in contrast to serum IgG levels observed with monthly IGIV treatment (rapid peaks followed by a slow decline).
14 CLINICAL STUDIES
14.1 US-Canada Study
The open-label, prospective, multicenter clinical study conducted in the US and Canada evaluated the pharmacokinetics, efficacy, safety, and tolerability of Vivaglobin in 65 (51 per-protocol) adult and pediatric subjects with PI. Subjects previously receiving monthly treatment with IGIV were switched to weekly subcutaneous administration of Vivaglobin for 12 months, after a 3-month wash-in/wash-out period.
The study evaluated the annual rate of serious bacterial infections (defined as bacterial pneumonia, meningitis, sepsis, osteomyelitis, and visceral abscesses). The annual rate of any infections was also evaluated.
In this study, the volume of Vivaglobin infused (using administration tubing and an infusion pump) did not exceed 15 mL per injection site at a rate of 20 mL per hour per site. Doses greater than 15 mL were divided and infused into multiple sites using Y-site connection tubing. For recommendations on the number of simultaneous injection sites for pediatric patients weighing less than 45 kg (99 pounds), see Administration (2.4).
Table 8 summarizes the dosing and annual rate of infections for the 51 per-protocol subjects in efficacy phase of the US-Canada study.
| bw, body weight. | |
| Number of per-protocol subjects (efficacy phase) | 51 |
| Weekly dose of Vivaglobin | |
| Mean | 158 mg/kg bw |
| Range | 34-352 mg/kg bw |
| Mean % previous IGIV dose (range) | 136%* (99%-188%) |
| Annual rate of serious bacterial infections | 0.04 infections/subject year†,‡ |
| Annual rate of any infections | 4.4 infections/subject year |
Table 9 provides a summary of missed school or work and hospitalization due to infection, which were also evaluated in the efficacy phase of the study.
| Number of per-protocol subjects (efficacy phase) | 51 |
| Total number of subject days | 18,949 |
| Total number of days missed school/work due to infection (%) | 192 (1.0%) |
| Annual rate missed school/work due to infection (days/subject year) | 3.70 |
| Total number of days hospitalized due to infection (%) | 12 (<0.1%) |
| Annual rate of hospitalization (days/subject year) | 0.23 |
14.2 Europe-Brazil Study
In a clinical study of Vivaglobin conducted in Europe and Brazil, 60 adult and pediatric subjects with PI were switched to weekly subcutaneous administration of Vivaglobin for 6 months. Forty-nine (49) subjects had been on IGIV, and 11 subjects had been treated long-term with another IGSC before entering the study. The 47 per-protocol subjects received a weekly mean dose of 89 mg/kg body weight of Vivaglobin (range: 51 to 147 mg/kg), which was 101% (range: 81% to 146%) of their previous immune globulin treatment.
The annualized rate of serious bacterial infections was 0.04 infections per subject year (one-sided upper 99% confidence interval: 0.21). The annualized rate of any infections was 4.3 infections per subject year).
15 REFERENCES
- Cayco AV, Perazella MA, Hayslett JP. Renal insufficiency after intravenous immune globulin therapy: a report of two cases and an analysis of the literature. J Am Soc Nephrol 1997;8:1788-1793.
- Dalakas MC. High-dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology 1994;44:223-226.
- Woodruff RK, Grigg AP, Firkin FC, Smith IL. Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet 1986;2:217-218.
- Wolberg AS, Kon RH, Monroe DM, Hoffman M. Coagulation factor XI is a contaminant in intravenous immunoglobulin preparations. Am J Hematol 2000;65:30-34.
- Gabor EP. Meningitis and skin reaction after intravenous immune globulin therapy. Ann Intern Med 1997;127:1130.
- Copelan EA, Strohm PL, Kennedy MS, Tutschka PJ. Hemolysis following intravenous immune globulin therapy. Transfusion 1986;26:410-412.
- Thomas MJ, Misbah SA, Chapel HM, Jones M, Elrington G, Newsom-Davis J. Hemolysis after high-dose intravenous Ig. Blood 1993;15:3789.
- Wilson JR, Bhoopalam N, Fisher M. Hemolytic anemia associated with intravenous immunoglobulin. Muscle Nerve 1997;20:1142-1145.
- Kessary-Shoham H, Levy Y, Shoenfeld Y, Lorber M, Gershon H. In vivo administration of intravenous immunoglobulin (IVIg) can lead to enhanced erythrocyte sequestration. J Autoimmun 1999;13:129-135.
- Rizk A, Gorson KC, Kenney L, Weinstein R. Transfusion-related acute lung injury after the infusion of IVIG. Transfusion 2001;41:264-268.
- Pierce LR, Jain N. Risks associated with the use of intravenous immunoglobulin. Trans Med Rev 2003;17:241-251.
- Smith GN, Griffiths B, Mollison D, Mollison PL. Uptake of IgG after intramuscular and subcutaneous injection. Lancet 1972;1:1208-1212.
- Waniewski I, Gardulf A, Hammarström L. Bioavailability of γ-globulin after subcutaneous infusions in patients with common variable immunodeficiency. J Clin Immunol 1994;14:90-97.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Vivaglobin is supplied in a single-use, tamper-evident vial containing 160 mg protein per mL of preservative-free liquid. Each vial label contains a peel-off strip with the vial size and product lot number for use in recording doses in a patient treatment record. The components used in the packaging for Vivaglobin are latex-free.
The following dosage forms are available:
| NDC Number | Vial Size | Packaging | Grams Protein |
|---|---|---|---|
| 0053-7596-01 | 3 mL | Single vial | 0.48 g |
| 0053-7596-10 | 10 mL | Single vial | 1.6 g |
| 0053-7596-20 | 20 mL | Single vial | 3.2 g |
17 PATIENT COUNSELING INFORMATION
17.1 Self-Administration
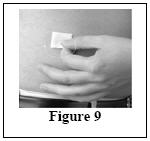
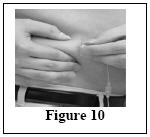
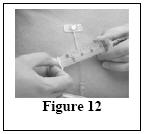
If self-administration is appropriate, ensure that the patient receives instructions and training on subcutaneous administration in the home setting. This should include the type of equipment to be used and information on its maintenance, demonstration of proper infusion techniques, selection of appropriate infusion sites (e.g., abdomen, thigh, upper arm, and/or lateral hip), maintenance of a treatment diary/log book, and measures to be taken in case of adverse reactions.
Make sure your patients understand how important it is that they adhere to the weekly administration schedule for Vivaglobin in order to maintain the steady levels of IgG in their blood. It is recommended that patients keep their treatment diary/log book current by recording, after each infusion, the time, date, dose, and any reactions, and by removing the peel-off portion of the label (containing the lot number) from the product vial and placing it in the treatment diary/log book.
Tell your patients that mild to moderate local injection-site reactions (e.g., swelling, redness, and itching) are a common side effect of subcutaneous therapy, but to contact their healthcare professional if a local reaction lasts longer than 4 days or is severe. With subcutaneous infusions, it is important that the needle is long enough to reach the subcutaneous tissue and that the actual point of injection be changed with each infusion.
17.2 Additional Information for Patients
- Dose adjustments – Tell patients that they may be tested regularly to make sure they have the correct levels of Vivaglobin (IgG) in their blood. These tests may result in adjustments to their dose.
- Hypersensitivity reactions – Inform patients of the early signs of hypersensitivity reactions to Vivaglobin (including hives, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis). Advise them of appropriate actions to take in the event of a hypersensitivity reaction.
- Interference with vaccines – Inform patients that administration of immunoglobulin may interfere with the response to live virus vaccines (e.g., measles, mumps, rubella, and varicella) and to notify their immunizing physician of recent therapy with Vivaglobin.
- Aseptic meningitis syndrome (AMS) – Inform patients of the symptoms of AMS, including severe headache, neck stiffness, drowsiness, fever, sensitivity to light, painful eye movements, nausea, and vomiting.
-
Reactions reported with IGIV treatment – Advise patients to be aware of and immediately report the following signs and symptoms to their healthcare professional:
- Decreased urine output, sudden weight gain, fluid retention/edema, and/or shortness of breath, which may suggest kidney problems.
- Shortness of breath, changes in mental status, chest pain, and other manifestations of thrombotic and embolic events.
- Fatigue, increased heart rate, yellowing of the skin or eyes, and dark-colored urine, which may suggest hemolysis.
- Severe breathing problems, lightheadedness, drops in blood pressure, and fever, which may suggest TRALI (a condition typically occurring within one to six hours following transfusion).
- Transmissible infectious agents – Inform patients that Vivaglobin is made from human plasma (part of the blood) and may contain infectious agents that can cause disease.
The attached Vivaglobin "Information for Patients" contains more detailed instructions for patients who will be self-administering Vivaglobin.
Vivaglobin (Pronounced VEE-vah-glow-bin)
Immune Globulin Subcutaneous (Human)
Information for patients
This leaflet contains important information about Vivaglobin. Please read it carefully before using this medicine. This information does not take the place of talking with your healthcare professional, and it does not include all of the available information about Vivaglobin. If you have any questions after reading this, ask your healthcare professional.
What is the most important information I should know about Vivaglobin?
- Vivaglobin is supposed to be infused under your skin only. DO NOT inject Vivaglobin into a blood vessel (vein or artery).
What is Vivaglobin?
Vivaglobin is a solution that contains proteins, called immunoglobulins, from plasma (the liquid part of human blood). These proteins are used to treat primary immunodeficiency (also called PI). People with primary immunodeficiency get a lot of infections because their immune system doesn't function properly.
Vivaglobin contains the antibody immunoglobulin G (IgG), which helps your body fight off infections caused by bacteria and viruses. Vivaglobin contains no preservatives, and the packaging contains no latex.
Who should NOT take Vivaglobin?
Do not take Vivaglobin if you have had a hypersensitivity reaction or a serious allergic reaction to other immunoglobulin medicines.
Tell your doctor if you have a condition called selective (or severe) immunoglobulin A (IgA) deficiency. This may mean you have a much greater chance of having an allergic reaction to Vivaglobin.
Tell your doctor if you have a history of heart or blood vessel disease or blood clots, have thick blood, or have been immobile for some time. These things may increase your risk of having a blood clot after using Vivaglobin.
How should I take Vivaglobin?
You will use a needle to infuse Vivaglobin under your skin. Do not use Vivaglobin until you have been taught how to infuse it. For the treatment to work properly, you must follow the dose and treatment schedule that your doctor gives you. Your doctor will obtain blood samples regularly to make sure you are getting the right amount of Vivaglobin.
Carefully read the "Instructions for Use" located at the end of this leaflet before you start your infusion. If you have any questions about the "Instructions for Use," discuss them with your doctor before you start to use Vivaglobin.
What should I avoid while taking Vivaglobin?
Vivaglobin can make some vaccines (like measles/mumps/rubella or chickenpox vaccines) not work as well for you. Before you get any vaccines, tell your doctor or healthcare professional that you take Vivaglobin.
Do not mix other products with the Vivaglobin solution.
Tell your doctor if you are pregnant or plan to become pregnant, or if you are nursing.
What are possible side effects of Vivaglobin?
The most common side effects with Vivaglobin occur in the area of the skin where the infusion needle is placed. These include redness, itching, swelling, and pain. Most of these side effects go away within 1 to 2 days. Tell your doctor if any of these side effects last for 4 days or more. These side effects may happen less and less with continued regular use of Vivaglobin.
Other side effects that might occur include:
- Headache/migraine
- Upset stomach
- Cramps
- Diarrhea
- Fever
- Nausea
- Sore throat
- Rash
- Pain
- Cough
- Fast heart rate
- Chest tightness
- Back pain
- Joint pain
- Muscle ache
Tell your doctor right away or go to the emergency room if you have hives, trouble breathing, wheezing, dizziness, or fainting. These could be signs of a bad allergic reaction.
Tell your doctor right away if you get any of the following symptoms. They could be signs of a serious problem.
- Reduced urination, sudden weight gain, or swelling in your legs. These could be signs of a kidney problem.
- Pain, swelling, warmth, redness, or a lump in your legs or arms. These could be signs of a blood clot.
- Bad headache with nausea, vomiting, stiff neck, fever, and sensitivity to light. These could be signs of a brain swelling called meningitis.
- Brown or red urine, fast heart rate, yellow skin or eyes. These could be signs of a blood problem.
- Chest pain or trouble breathing.
- Fever over 100°F. This could be a sign of an infection.
Tell your doctor about any side effects that concern you. You can ask your doctor to give you more information that is available to healthcare professionals.
How do I use Vivaglobin?
Vivaglobin is infused under the skin (subcutaneously). With proper training, you can infuse it on your own. Do not inject Vivaglobin into a blood vessel (vein or artery).
Your doctor will determine the appropriate dose and schedule for your treatment.
Your healthcare professional will teach you how to infuse Vivaglobin. Always follow the instructions you receive. The instructions below are general guidelines for using Vivaglobin. Use them only as an aid once you have learned the proper way to infuse Vivaglobin. Call your healthcare professional if you are not sure about the procedure or if you have any questions about these instructions.
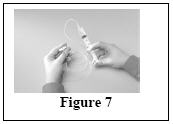
Instructions for use
Do not use Vivaglobin until you have been taught how to use it by your doctor. For the treatment to work, you must carefully follow your doctor's instructions. The instructions below are provided to you as a guide. If you have any questions about these instructions, it is important that you discuss them with your doctor before you start to use Vivaglobin.
Keep Vivaglobin and all other medicines away from children.
Vivaglobin comes in single-use vials. Keep these vials in their storage box in the refrigerator at 2-8°C (36-46°F) until you are ready to use them. Do not freeze. Vivaglobin has no preservatives added, so it is important to throw away any Vivaglobin leftover in a vial after it is opened.
|
|
|  |
|  |
|  |
|  |
|  |
|  |
|  |
|   |
|  |
|  |
|  |
|  |
|  |
|  |
|
|
Be sure to tell your doctor about any problems you have doing your infusions. Your doctor may ask to see your treatment diary or logbook, so be sure to take it with you each time you visit the doctor's office.
Call your doctor for medical advice about side effects. You can also report side effects to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Manufactured by:
CSL Behring GmbH
35041 Marburg, Germany
US License No. 1765
Distributed by:
CSL Behring LLC
Kankakee, IL 60901 USA
| VIVAGLOBIN
human immunoglobulin g solution |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - CSL Behring LLC (058268293) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CSL Behring GmbH | 326530474 | MANUFACTURE(0053-7596) | |