KOPERTOX- copper naphthenate liquid
Fort Dodge Animal Health Div of Wyeth
----------
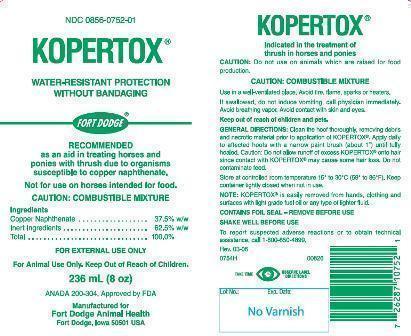
KOPERTOX®
WATER-RESISTANT PROTECTION WITHOUT BANDAGING
RECOMMENDED as an aid in treating horses and ponies with thrush due to organisms susceptible to copper naphthenate.
Inert Ingredients............................................ 62.5% w/w
Total............................................................... 100%
CAUTION: Do not use in horses intended for human consumption.
CAUTION: COMBUSTIBLE MIXTURE
Use in a well-ventilated place. Avoid fire, flame, sparks or heaters.
If swallowed, do not induce vomiting, call physician immediately. Avoid breathing vapor. Avoid contact with skin and eyes.
Keep out of reach of children and pets.
GENERAL DIRECTIONS:
Clean the hoof thoroughly, removing debris and necrotic material prior to application of KOPERTOX®. Apply daily to affected hoofs with a narrow paint brush (about 1") until fully healed. Caution: Do not allow runoff of excess KOPERTOX® onto hair since contact with KOPERTOX® may cause some hair loss. Do not contaminate feed.
Store at controlled room temperature 15° to 30°C (59° to 86°F). Keep container tightly closed when not in use.
NOTE: KOPERTOX® is easily removed from hands, clothing and surfaces with light grade fuel oil or any type of lighter fluid.
CONTAINS FOIL SEAL – REMOVE BEFORE USE.
SHAKE WELL BEFORE USE.
| KOPERTOX
copper naphthenate liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Fort Dodge Animal Health Div of Wyeth (149957656) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| FIRST PRIORITY INCORPORATED | 179925722 | manufacture | |