COATED ASPIRIN LOW-DOSE- aspirin tablet, delayed release
Target Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Target 44-600A Delisted
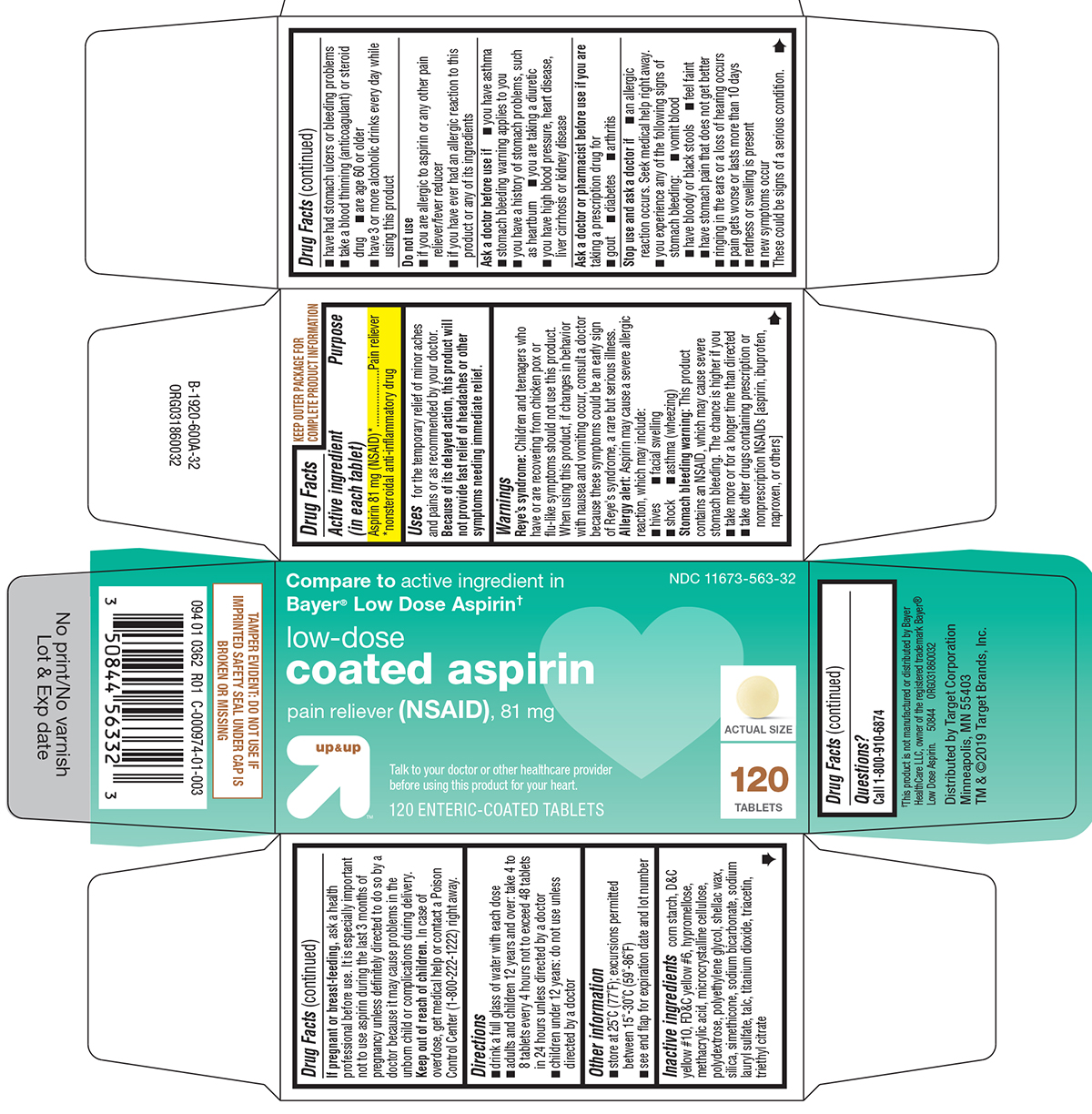
Uses
for the temporary relief of minor aches and pains or as recommended by your doctor. Because of its delayed action, this product will not provide fast relief of headaches or other symptoms needing immediate relief.
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include:
- hives
- facial swelling
- shock
- asthma (wheezing)
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- are age 60 or older
- take more or for a longer time than directed
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- have 3 or more alcoholic drinks every day while using this product
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
Do not use
- if you are allergic to aspirin or any other pain reliever/fever reducer
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis or kidney disease
- you have asthma
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
taking a prescription drug for
- gout
- diabetes
- arthritis
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away.
- you experience any of the following signs of stomach bleeding:
- vomit blood
- have bloody or black stools
- feel faint
- have stomach pain that does not get better
- ringing in the ears or a loss of hearing occurs
- pain gets worse or lasts more than 10 days
- redness or swelling is present
- new symptoms occur
These could be signs of a serious condition.
Directions
- drink a full glass of water with each dose
- adults and children 12 years and over: take 4 to 8 tablets every 4 hours not to exceed 48 tablets in 24 hours unless directed by a doctor
- children under 12 years: do not use unless directed by a doctor
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, D&C yellow #10, FD&C yellow #6, hypromellose, methacrylic acid, microcrystalline cellulose, polydextrose, polyethylene glycol, shellac wax, silica, simethicone, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triacetin, triethyl citrate
Principal Display Panel
NDC 11673-563-32
Compare to active ingredient in
Bayer® Low Dose Aspirin†
low-dose
coated aspirin
pain reliever (NSAID), 81 mg
up & up™
Talk to your doctor or other healthcare provider
before using this product for your heart.
120 ENTERIC-COATED TABLETS
ACTUAL SIZE
120
TABLETS
TAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER CAP IS
BROKEN OR MISSING
†This product is not manufactured or distributed by Bayer
HealthCare LLC, owner of the registered trademark Bayer®
Low Dose Aspirin. 50844 ORG031860032
Distributed by Target Corporation
Minneapolis, MN 55403
TM & ©2019 Target Brands, Inc.
Target 44-600A
| COATED ASPIRIN
LOW-DOSE
aspirin tablet, delayed release |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Target Corporation (006961700) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | manufacture(11673-563) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(11673-563) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | manufacture(11673-563) , pack(11673-563) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 117025878 | manufacture(11673-563) | |