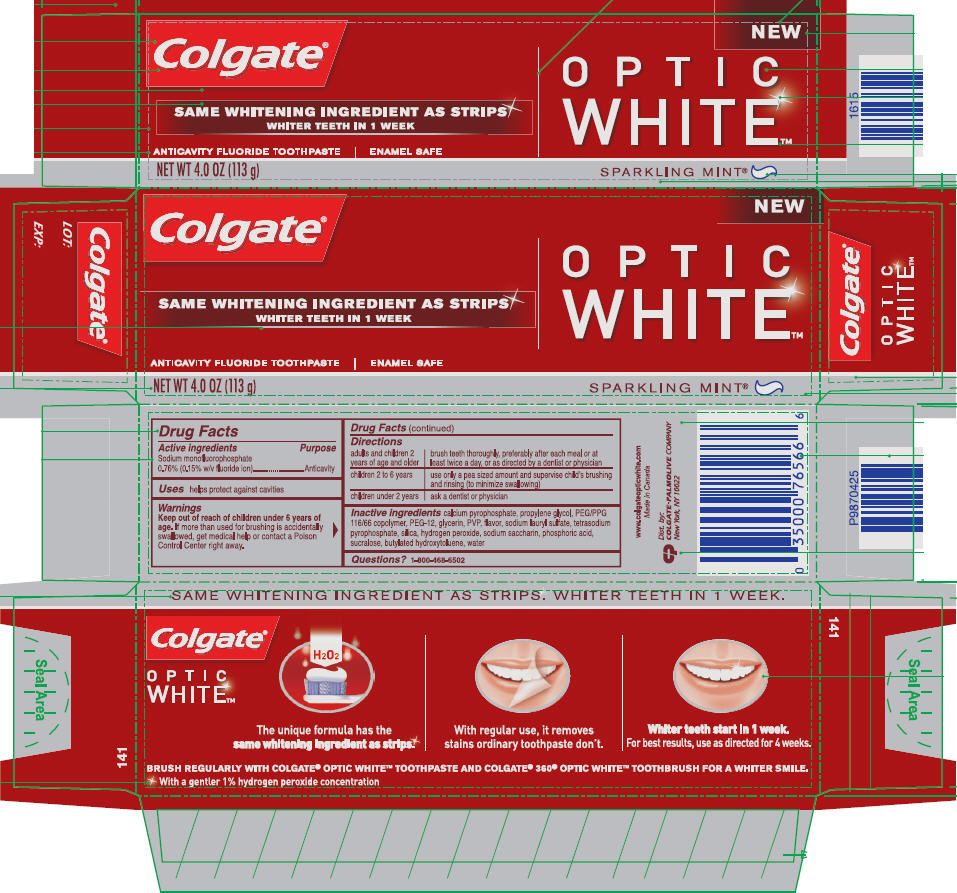
COLGATE OPTIC WHITE - SPARKLING MINT- sodium monofluorophosphate paste, dentifrice
Colgate-Palmolive Canada
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Colgate®
OPTIC
WHITE™
Directions
| adults and children 2 years of age and older | brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician |
| children 2 to 6 years | use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) |
| children under 2 years | ask a dentist or physician |
| COLGATE
OPTIC WHITE - SPARKLING MINT
sodium monofluorophosphate paste, dentifrice |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Colgate-Palmolive Canada (259157246) |
Revised: 9/2019
Document Id: 71305dc6-849b-4f80-b1b1-f4162800192e
Set id: 8698e7f7-582a-407f-a5d8-17a37ba2c2de
Version: 2
Effective Time: 20190909
Colgate-Palmolive Canada