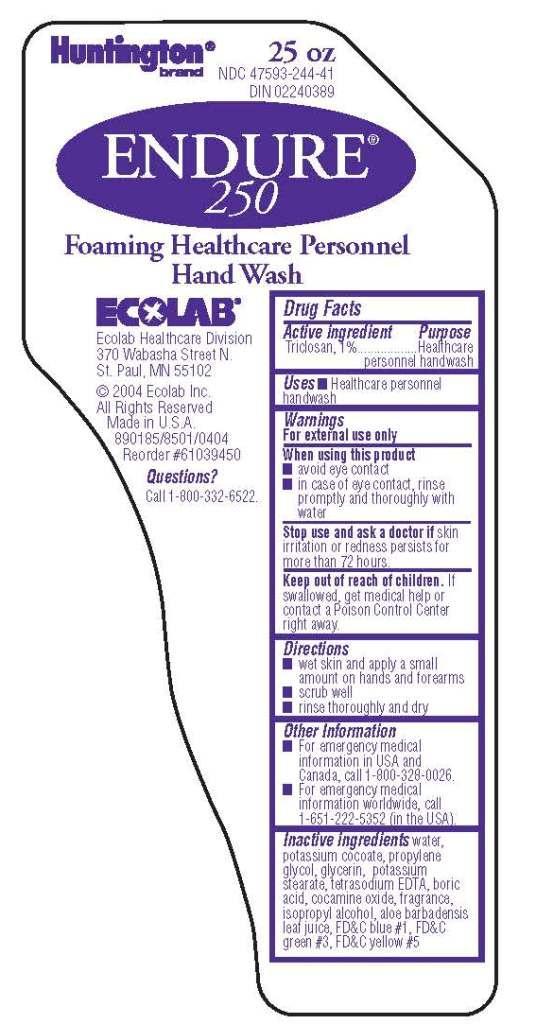
ENDURE 250- triclosan solution
Ecolab Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Warnings
For external use only
Directions
- wet skin and apply a small amount on hands and forearms
- scrub well
- rinse thoroughly and dry
Other Information
- For emergency medical information in USA and Canada, call 1-800-328-0026.
- For emergency medical information worldwide, call 1-651-222-5352 (in the USA).
| ENDURE 250
triclosan solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Ecolab Inc. (006154611) |
Revised: 1/2018
Document Id: f863d7f2-420d-412f-8518-4af0112d8b1c
Set id: 8408b8e5-546f-4228-b17d-7105fc32cd7a
Version: 4
Effective Time: 20180129
Ecolab Inc.