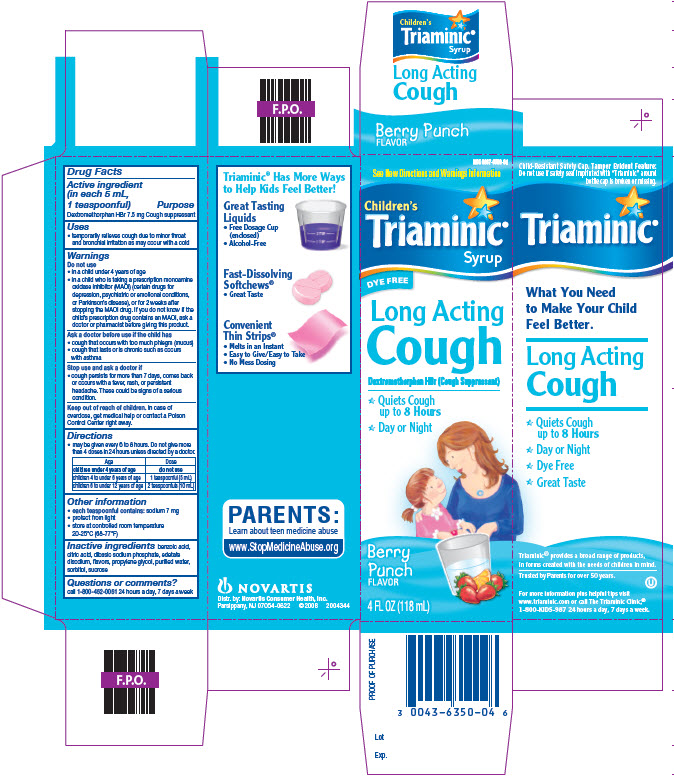
TRIAMINIC LONG ACTING COUGH- dextromethorphan hbr syrup
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
● temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
Warnings
Click here to enter Warnings
Do Not Use
● in a child under 4 years of age
● in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s Disease), or for 2 weeks after stopping the MAOI drug. If you do not know if the child’s prescription contains an MAOI, ask a doctor or pharmacist before giving this product
Directions
● may be given every 6 to 8 hours. Do not give more than 4 doses in 24 hours unless directed by a doctor.
Other information
● each teaspoonful contains: sodium 7 mg
● protect from light
● store at controlled room temperature 20-25°C (68-77°F)
| TRIAMINIC LONG ACTING COUGH
dextromethorphan hbr syrup |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |