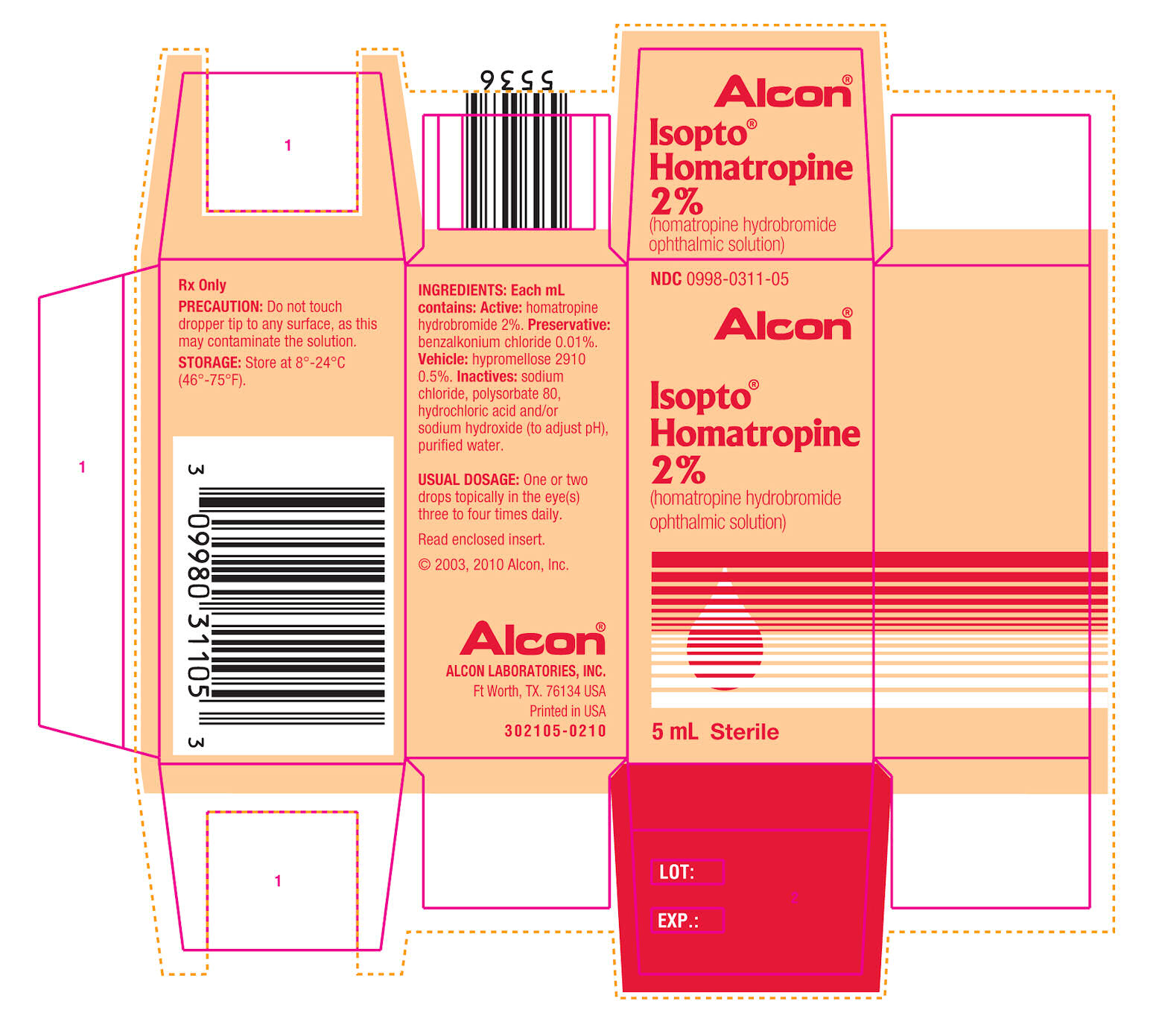
ISOPTO HOMATROPINE - homatropine hydrobromide solution
Alcon Laboratories, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Isopto® Homatropine
(homatropine hydrobromide ophthalmic solution)
Sterile
DESCRIPTION SECTION
ISOPTO® Homatropine (homatropine hydrobromide ophthalmic solution) is an anticholinergic prepared as a sterile topical ophthalmic solution supplied in two strengths. The active ingredient is represented by the chemical structure:
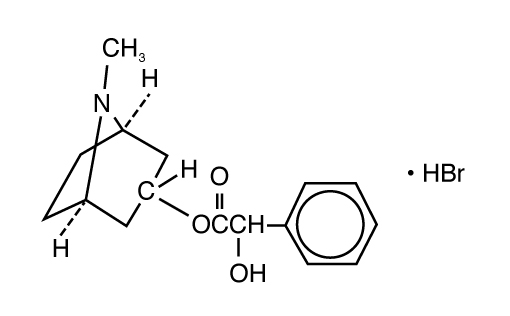
Established name: Homatropine Hydrobromide
Chemical name: Benzeneacetic acid, α-hydroxy-, 8-methyl-8-azabicyclo[3.2.1]-oct-3-yl ester, hydrobromide, endo-(±)-.
Each mL contains: Active: homatropine hydrobromide 2.0% or 5.0%. Preservatives: benzalkonium chloride 0.01% in 2% strength, benzethonium chloride 0.005% in 5% strength. Vehicle: hypromellose 0.5%. Inactives: sodium chloride, polysorbate 80 (in 2% strength), sodium hydroxide and/or hydrochloric acid (to adjust pH), purified water.
CLINICAL PHARMACOLOGY SECTION
This anticholinergic preparation blocks the responses of the sphincter muscle of the iris and the accommodative muscle of the ciliary body to cholinergic stimulation, producing pupillary dilation (mydriasis) and paralysis of accommodation (cycloplegia).
INDICATIONS & USAGE SECTION
A moderately long-acting mydriatic and cycloplegic for cycloplegic refraction and in the treatment of inflammatory conditions of the uveal tract. For pre- and postoperative states when mydriasis is required. Use as an optical aid in some cases of axial lens opacities.
CONTRAINDICATIONS SECTION
Contraindicated in persons with primary glaucoma or a tendency toward glaucoma, e.g., narrow anterior chamber angle, and in those persons showing hypersensitivity to any component of this preparation.
WARNINGS SECTION
FOR TOPICAL OPHTHALMIC USE ONLY - NOT FOR INJECTION. Risk-benefit should be considered when the following medical problems exist: keratoconus (homatropine may produce fixed dilated pupil); Down’s syndrome, children with brain damage and the elderly (increased susceptibility). In infants and small children, use with extreme caution.
PRECAUTIONS SECTION
General
To avoid excessive systemic absorption, the lacrimal sac should be compressed by digital pressure for two to three minutes after instillation. To avoid inducing angle closure glaucoma, an estimation of the depth of the angle of the anterior chamber should be made. Excessive topical use of this drug can potentially lead to a confusional state characterized by delirium, agitation, and rarely coma. This state is more apt to occur in the pediatric and geriatric age groups. The specific antidote for this systemic anticholinergic syndrome is injectable physostigmine salicylate.
Information for Patients
Patient should be advised not to drive or engage in other hazardous activities while pupils are dilated. Patient may experience sensitivity to light and should protect eyes in bright illumination during dilation. Parents should be warned not to get this preparation in their child’s mouth and to wash their own hands and the child’s hands following administration. Do not touch dropper tip to any surface, as this may contaminate the solution.
Carcinogenesis, Mutagenesis, Impairment of Fertility
There have been no long-term studies done using homatropine hydrobromide in animals to evaluate carcinogenic potential.
Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with homatropine hydrobromide. It is also not known whether homatropine hydrobromide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Homatropine hydrobromide should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS SECTION
Transient symptoms of stinging and burning may occur. Prolonged use may produce local irritation characterized by follicular conjunctivitis, vascular congestion, edema, exudate, and an eczematoid dermatitis. Thirst or dryness of mouth, eye irritation not present before therapy, or increased sensitivity of eyes to light may occur.
Overdose for Homatropine
Systemic homatropine toxicity is manifested by flushing and dryness of the skin (a rash may be present in children), blurred vision, a rapid and irregular pulse, fever, abdominal distention in infants, mental aberration (hallucinosis) and loss of neuro-muscular coordination. Atropine poisoning, although distressing, is rarely fatal even with large doses of atropine, and is self-limited if the cause is recognized and the homatropine medication is discontinued. Treatment includes supportive measures including maintaining a patent airway and assisting respiration if needed. Treat hyperthermia, coma and seizures if they occur (1). In infants and children, the body surface must be kept moist. Excitement may be controlled by diazepam or a short-acting barbiturate. For ingestion, activated charcoal can be used to prevent drug absorption. If necessary, ipecac or another cathartic may be useful for drug removal during initial treatment (1, 2). Physostigmine is used as an antidote to the systemic effects of atropine and may be administered parenterally to provide more prompt relief of intoxication. Parenteral physostigmine may be particularly useful in cases of pronounced hallucinations, agitation in which a patient may be dangerous to himself or others, arrhythmias resulting in uncontrolled hemodynamic instability, and intractable seizures.
DOSAGE & ADMINISTRATION SECTION
For refraction, instill one or two drops topically in the eye(s). May be repeated in five to ten minutes if necessary. For uveitis, instill one or two drops topically up to every three to four hours. Individuals with heavily pigmented irides may require larger doses. Only the 2% strength should be used in pediatric patients.
HOW SUPPLIED SECTION
5 mL in plastic DROP-TAINER® Dispensers.
2% ISOPTO® Homatropine
5 mL NDC 0998-0311-05
5% ISOPTO® Homatropine
5 mL NDC 0998-0315-05
REFERENCES SECTION
- Kirk M, Kulig K, Rumack BH. Anticholinergics. In: Clinical Management of Poisoning and Drug Overdose, Second Edition. Edited by Haddad LM, Winchester JF. Philadelphia, W.B. Sauders Company, 1990, p 861-867.
- Tani SA. Anticholinergics. In: Poisoning and Drug Overdose, Second Edition. Olson KR. Norwalk, CT, Appleton & Lange, 1994, p 75-76.
Revised: June 2007
Printed in USA
9002668-0607
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
© 2002, 2003, 2007 Alcon, Inc.
| ISOPTO HOMATROPINE
homatropine hydrobromide solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| ISOPTO HOMATROPINE
homatropine hydrobromide solution |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Alcon Laboratories, Inc. (008018525) |
| Registrant - Alcon Laboratories, Inc. (008018525) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Alcon Research Ltd | 007672236 | MANUFACTURE(0998-0311) | |