OCEAN HAND SANITIZER- alcohol liquid
Mangiacotti, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
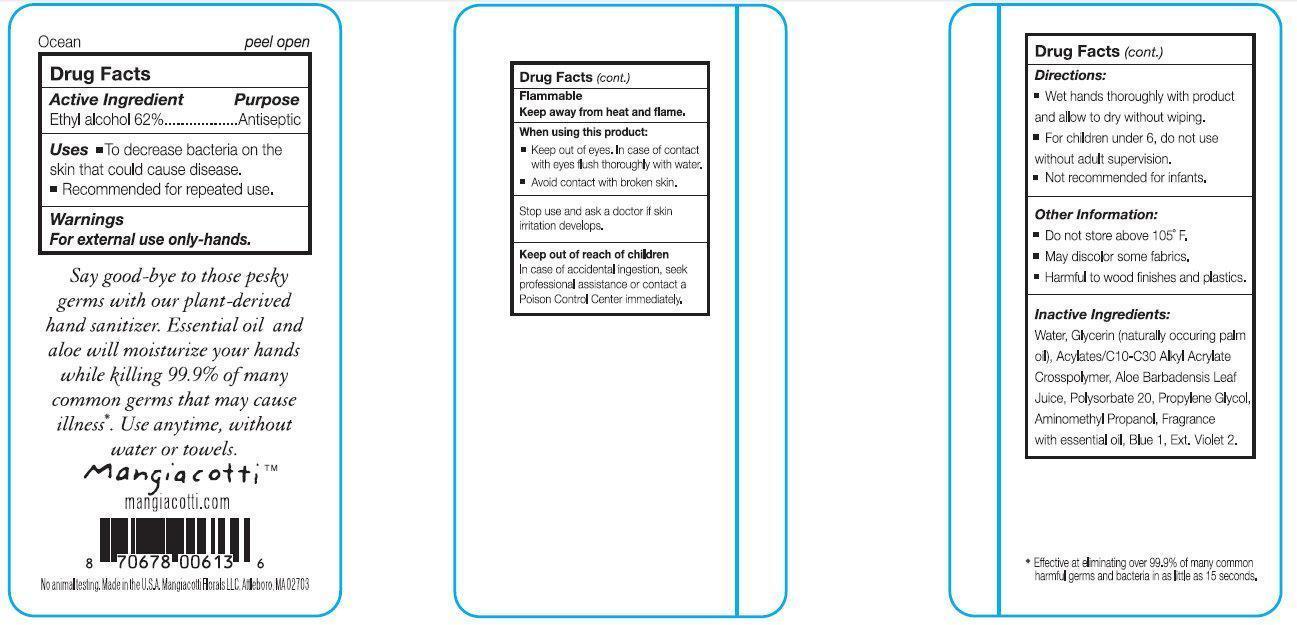
Ocean Hand Sanitizer
WARNINGS
For external use only - hands.
DIRECTIONS:
- Wet hands thoroughly with product and allow to dry without wiping.
- For children under 6, do not use without adult supervision.
- Not recommended for infants.
OTHER INFORMATION:
- Do not store above 105 degrees F.
- May discolor some fabrics.
- Harmful to wood finishes and plastics.
INACTIVE INGREDIENTS
Water, Glycerin (naturally occurring palm oil), Acrylates/C10-C30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, Polysorbate 20, Propylene Glycol, Aminomethyl Propanol, Fragrance with essential oil, Blue 1, Ext.Violet 2.
Say good-bye to those pesky germs with out plant-derived hand sanitizer. Essential oil and aloe will moisturize your hands while killing 99.9% of many common germs that may cause illness*.
Use anytime, without water or towels.
Mangiacotti
mangiacotti.com
* Effective at eliminating over 99.9% of many common harmful germs and bacteria in as little as 15 seconds.
No animal testing. MADE IN USA
Mangiacotti Florals LLC
| OCEAN HAND SANITIZER
alcohol liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Mangiacotti, Inc (078850804) |
| Registrant - Harrison Specialty Co., Inc. (001000355) |