Label: GRANULOTION- menthol, zinc oxide lotion
- NDC Code(s): 65121-885-23
- Packager: Pure Source, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- GranuLotion medicated lotion
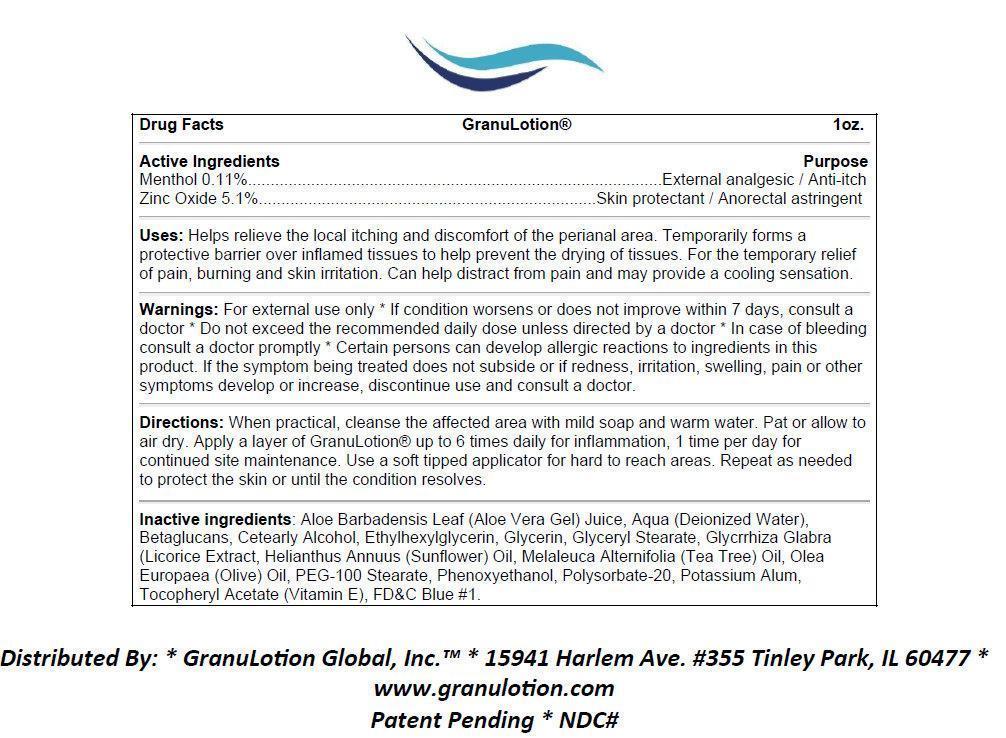
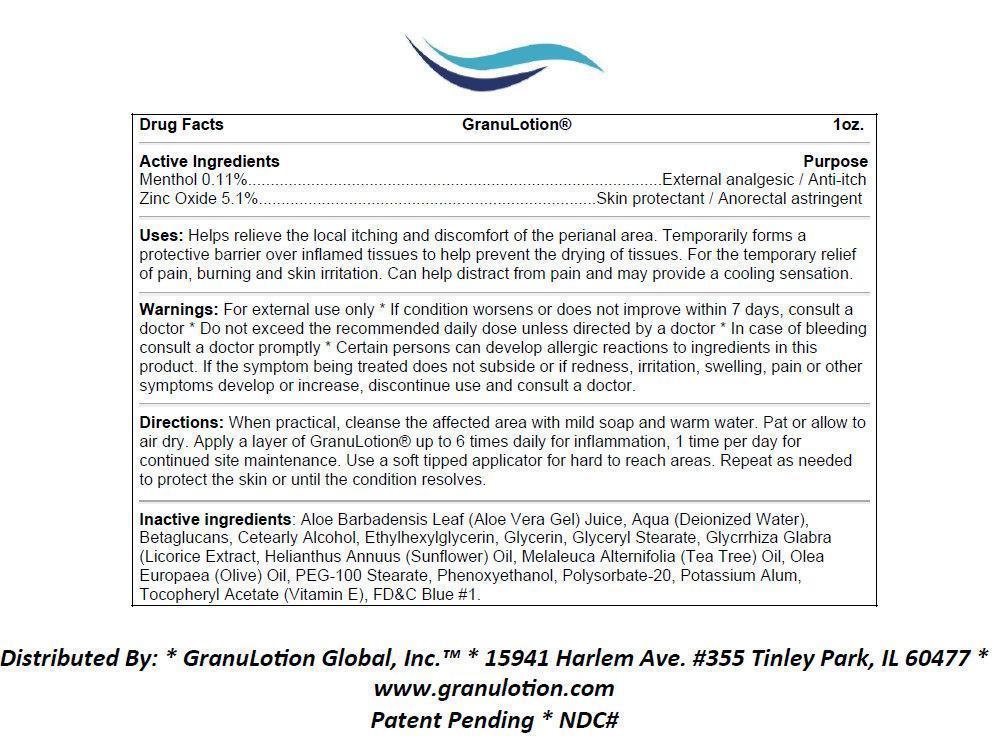
- Active Ingredients
- Purpose
- Uses:
-
Warnings
For external use only * If condition worsens or does not improve within 7 days, consult a doctor * Do not exceed the recommended daily dose unless directed by a doctor * In case of bleeding consult a doctor promptly * Certain persons can develop allergic reactions to ingredients in this product. If the symptom being treated does not subside or if redness, irritation, swelling, pain or other symptoms develop or increase, discontinue use and consult a doctor.
-
Directions:
When practical, cleanse the affected area with mild soap and warm water. Pat or allow to air dry. Apply a layer of GranuLotion® up to 6 times daily for inflammation, 1 time per day for continued site maintenance. Use a soft tipped applicator for hard to reach areas. Repeat as needed to protect the skin or until the condition resolves.
-
Inactive Ingredients:
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Betaglucans, Cetearly Alcohol, Ethylhexylglycerin, Glycerin, Glyceryl Stearate, Glycrrhiza Glabra (Licorice Extract, Helianthus Annuus (Sunflower) Oil, Melaleuca Alternifolia (Tea Tree) Oil, Olea Europaea (Olive) Oil, PEG-100 Stearate, Phenoxyethanol, Polysorbate-20, Potassium Alum, Tocopheryl Acetate (Vitamin E), FDandC Blue 1.
- GranuLotion medicated lotion 1oz/28.349g (65121-885-23)
-
INGREDIENTS AND APPEARANCE
GRANULOTION
menthol, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65121-885 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.11 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 5.1 g in 100 g Inactive Ingredients Ingredient Name Strength TEA TREE OIL (UNII: VIF565UC2G) OLIVE OIL (UNII: 6UYK2W1W1E) PEG-100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) POTASSIUM ALUM (UNII: 1L24V9R23S) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LICORICE (UNII: 61ZBX54883) SUNFLOWER OIL (UNII: 3W1JG795YI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65121-885-23 1 in 1 BOX 02/09/2017 1 28.349 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 03/26/2014 Labeler - Pure Source, LLC (080354456) Establishment Name Address ID/FEI Business Operations Pure Source, LLC 080354456 manufacture(65121-885)