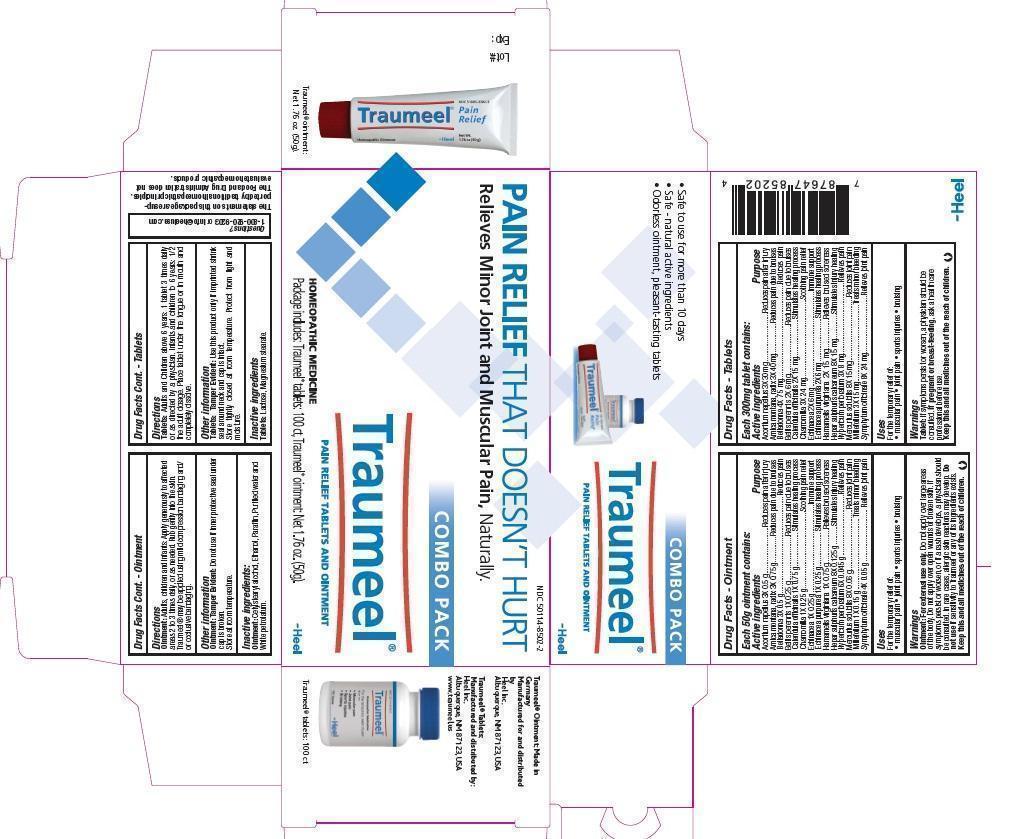
ACTIVE INGREDIENTS
Traumeel Tablet:
Each 300 mg tablet contains as active ingredients: Aconitum napellus 3X 30 mg; Arnica montana, radix 3X 40 mg; Belladonna 4X 75 mg; Bellis perennis 2X 6 mg; Calendula officinalis 2X 15 mg; Chamomilla 3X 24 mg; Echinacea 2X 6 mg; Echinacea purpurea 2X 6 mg; Hamamelis virginiana 2X 15 mg; Hepar sulphuris calcareum 8X 15 mg; Hypericum perforatum 3X 8 mg; Mercurius solubilis 8X 15 mg; Millefolium 3X 15 mg; Symphytum officinale 8X 24 mg.
Traumeel Ointment:
Each 50g of ointment contains: Calendula officinalis 1X, Hamamelis virginiana 1X, Arnica montana, radix 3X 0.75 g each; Aconitum napellus 3X, Belladonna 3X 0.5 g each; Bellis perennis 1X, Chamomilla 1X, Echinacea 1X, Echinacea purpurea 1X 0.25 g each; Millefolium 1X 0.15 g; Hepar sulphuris calcareum 8X 0.125 g; Mercurius solubilis 8X 0.06g; Symphytum officinale 4X 0.05 g Hypericum perforatum 6X 0.045 g.
PURPOSE
Traumeel Tablet:
Aconitum napellus 3X 30 mg.................Reduces pain after injury Arnica montana, radix 3X 40 mg............Reduces pain due to bruises Belladonna 4X 75 mg............................Reduces pain Bellis perennis 2X 6 mg........................Reduces pain due to bruises Calendula officinalis 2X 15 mg..............Stimulates healing process Chamomilla 3X 24 mg..........................Soothing pain relief Echinacea 2X 6 mg..............................Immune support Echinacea purpurea 2X 6 mg................Stimulates healing process Hamamelis virginiana 2X 15 mg............Relieves bruised soreness Hepar sulphuris calcareum 8X 15 mg....Stimulates injury healing Hypericum perforatum 3X 8 mg.............Relieves pain Mercurius solubilis 8X 15 mg................Reduces joint pain Millefolium 3X 15 mg...........................Treats minor bleeding Symphytum officinale 8X 24 mg...........Relieves joint pain
Traumeel Ointment:
Calendula officinalis 1X 1.5 g....................Stimulates healing process Hamamelis virginiana 1X 1.5 g.................Relieves bruised soreness Arnica montana, radix 3X 1.5 g.................Reduces bruising Aconitum napellus 3X 1 g.........................Reduces pain after injury Belladonna 3X 1 g....................................Reduces pain Bellis perennis 1X 0.5 g............................Treats bruises Chamomilla 1X 0.5 g................................Soothing pain relief Echinacea 1X 0.5 g..................................Immune support Echinacea purpurea 1X 0.5 g....................Stimulates healing process Millefolium 1X 0.3 g.................................Treats minor bleeding Hepar sulphuris calcareum 8X 0.25 g........Stimulates injury healing Mercurius solubilis 8X 0.12 g....................Reduces joint pain Symphytum officinale 4X 0.1 g..................Relieves joint pain Hypericum perforatum 6X 0.09 g...............Relieves pain
KEEP OUT OF REACH OF CHILDREN
Traumeel Tablets: Keep this and all medicine out of the reach of children.
Traumeel Ointment: Keep this and all medicine out of the reach of children.
INDICATIONS AND USAGE
Traumeel Tablets:
For the temporary relief of pain due to:Muscular painJoint painSports injuriesBruising
Traumeel Ointment:
For the temporary relief of muscular pain, joint pain, sports injuries and bruising.
WARNINGS
Traumeel Tablets:
If symptoms persist or worsen, a physician should be consulted. If pregnant or breast-feeding, ask a health care professional before use.
Traumeel Ointment:
For external use only. Do not apply over large areas of the body. Do not apply over open wounds or broken skin. If symptoms persist or worsen, or if a rash develops, a physician should be consulted. In rare cases, allergic skin reactions may develop. Do not use if known sensitivity to Traumeel or any of its ingredients exists.
DOSAGE AND ADMINISTRATION
Traumeel Tablets:
Adults and children above 6 years: 1 tablet 3 times daily or as directed by a physician. Infants and children to 6 years: 1/2 the adult dosage. Place tablet under the tongue or in mouth and completely dissolve.
Traumeel Ointment:
Adults, children and infants: Apply generously to affected areas 2 to 3 times daily, or as needed. Rub gently into the skin. Traumeel may be applied using mild compression bandaging and/or occlusive bandaging.
INACTIVE INGREDIENTS
Traumeel Tablets:
Lactose, Magnesium stearate
Traumeel Ointment:
Cetylstearyl alcohol, Ethanol, Parafin, Purified water, White petrolatum