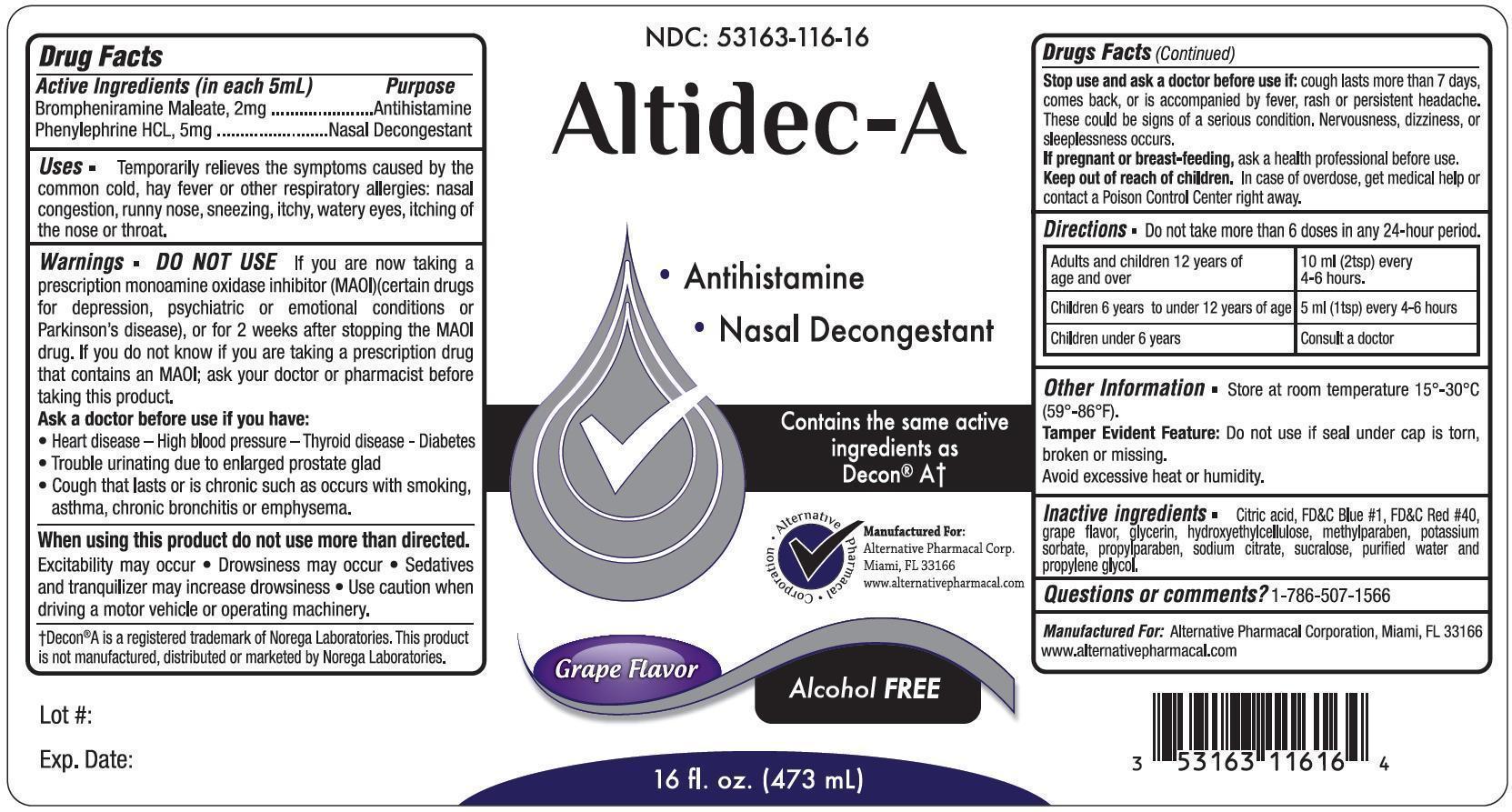
ALTIDEC A- brompheniramine maleate, phenylephrine hcl liquid
Alternative Pharmacal Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients (in each 5 mL) Purpose
Brompheniramine Maleate ............. 2 mg ........ Antihistamine
Phenylephrine HCL .................. 5 mg ...... Nasal Decongestant
Uses
Temporarily relieves the symptoms caused by the common cold, hay fever or other respiratory allergies; nasal congestrion, runny nose, sneezing, itchy, watery eyes, itching of the nose or throat.
Warnings
DO NOT USE
If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if you are taking a prescription drug that contains MAOI; ask your doctor or pharmacist before taking this product.
Ask a doctor before use if you have:
- Heart disease - High blood pressure - thyroid deases - diabetes
- Trouble urinating due to enlarged prostate gland
- Cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema.
When using this product do not use more than directed.
- excitability may occur
- drowsiness may occur
- sedatives and tranquilizer may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
When using this prouct do not use more than directed.
Stop use and ask a doctor before use if: cough lasts more than 7 days, comes back, or is accompanied by fever, rash or persistent headache. These could be signs of a serious condition. Nervouseness, dizziness or sleeplessness occurs.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions Do not take more than 6 doses in any 24-hour period.
| Adults and children 12 years of age and over | 10 ml (2 tsp) every 4 - 6 hours |
| Children 6 years to under 12 years of age | 5 ml (1 tsp) every 4 - 6hours |
| Children under 6 years of age | consult a doctor |
Other Information
Store at room temperature 150 - 300C (590-860F)
Tamper Evident Feature: Do not use if seal under cap is torn, broken or missing. Avoid excessive heat or humidity.
| ALTIDEC
A
brompheniramine maleate, phenylephrine hcl liquid |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Alternative Pharmacal Corporation (078528214) |