Label: MEIJER- mineral oil enema
-
Contains inactivated NDC Code(s)
NDC Code(s): 41250-112-11 - Packager: Meijer Distribution Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 2, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For rectal use only
Ask a doctor before use if you have
- •
- abdominal pain, nausea or vomiting
- •
- notice a sudden change in bowel habits that persists over a period of 2 weeks
-
Directions
Single daily dose
adults & children
12 years and over
1 bottle once daily
children 2 to under
12 years
1/2 bottle once daily
Discard unused portion
children under
2 years
do not use
How to use this enema:
REMOVE GREEN PROTECTIVE SHIELD BEFORE INSERTING; HOLD BOTTLE UPRIGHT, GRASPING BOTTLE CAP WITH FINGERS. GRASP PROTECTIVE SHIELD WITH OTHER HAND AND PULL GENTLY TO REMOVE.
Administering enema:
- •
- With steady pressure, gently insert enema tip into rectum with a slight side-to-side movement, with tip pointing toward navel. Insertion may be easier if person receiving enema bears down, as if having a bowel movement. This helps relax the muscles around the anus.
- •
- Do not force the enema tip into rectum as this can cause injury.
- •
- Squeeze bottle until nearly all liquid is gone. It is not necessary to empty the bottle completely, as it contains more liquid than needed.
- •
- Remove enema tip from rectum.
- •
- Retain enema in accordance with doctor's instructions.
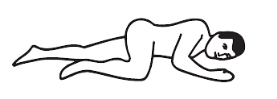
Positioning:
Left-side position: Lie on left side with knee bent and arms at rest.
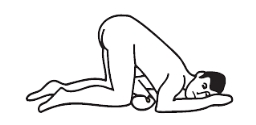
Knee-chest position:
Kneel, then lower head and chest forward until left side of face is resting on surface. Position arms comfortably.
- Other Information
- Inactive ingredient
- Questions or comments?
-
Principal Display Panel
NDC 41250-112-11
Compare to Fleet® Mineral Oil Enema active ingredient*
Meijer
Ready-To-Use
Enema Mineral Oil
Sodium Free
Lubricant Laxative
For Relief of
Occasional Constipation
Latex Free
Protective Shield prevents contamination
Safety Seal for added protection
Comfortable, lubricated tip for insertion
One-way safety valve controls flow and prevents reflux
Easy, soft squeeze bottle
4.5 FL OZ (133ml)
- Back & Side Panel - Box
-
INGREDIENTS AND APPEARANCE
MEIJER
mineral oil enemaProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41250-112 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINERAL OIL (UNII: T5L8T28FGP) (MINERAL OIL - UNII:T5L8T28FGP) MINERAL OIL 113.115 g in 118 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41250-112-11 1 in 1 BOX 1 133 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 05/10/2013 Labeler - Meijer Distribution Inc (006959555)