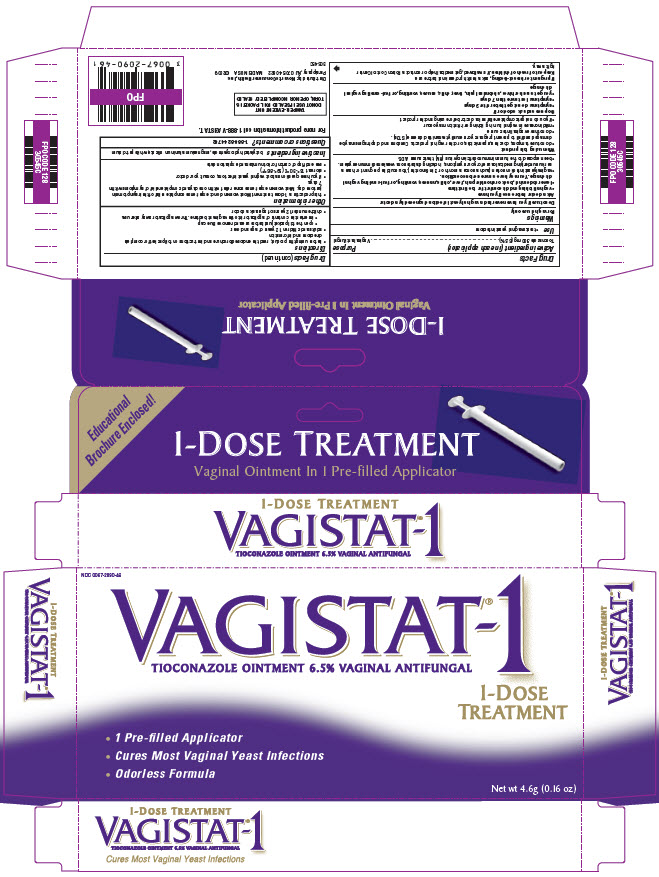
VAGISTAT-1- tioconazole ointment
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
----------
Drug Facts
Warnings
For vaginal use only
Do not use if you have never had a vaginal yeast infection diagnosed by a doctor
[Enter Do Not Use here]
Ask a doctor before use if you have
- •
- vaginal itching and discomfort for the first time
- •
- lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge. You may have a more serious condition.
- •
- vaginal yeast infections often (such as once a month or 3 in 6 months). You could be pregnant or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
- •
- been exposed to human immunodeficiency virus (HIV) that causes AIDS
When using this product
- •
- do not use tampons, douches, spermicides, or other vaginal products. Condoms and diaphragms may be damaged and fail to prevent pregnancy or sexually transmitted disease (STDs).
- •
- do not have vaginal intercourse
- •
- mild increase in vaginal burning, itching or irritation may occur
- •
- if you do not get complete relief ask a doctor before using another product
Directions
- •
- before using this product, read the enclosed brochure and instructions on foil packet for complete directions and information
- •
- adults and children 12 years of age and over:
- •
- open the foil packet just before use and remove blue cap
- •
- insert entire contents of applicator into the vagina at bedtime. Throw applicator away after use.
- •
- children under 12 years of age: ask a doctor
Other information
- •
- this product is a 1-dose treatment. Most women do not experience complete relief of their symptoms in just one day. Most women experience some relief within one day and complete relief of symptoms within 7 days.
- •
- if you have questions about vaginal yeast infections, consult your doctor
- •
- store at 15° - 30°C (59° - 86°F)
- •
- see end flap of carton for lot number and expiration date
| VAGISTAT-1
tioconazole ointment |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |
Revised: 12/2016
Document Id: 60b2a2db-9bfa-4904-bd50-021f000c8ac1
Set id: 78ebebac-fc6a-42b9-a402-8a830c51a043
Version: 2
Effective Time: 20161205
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC