NOTAFLU LEMON OIL- lemon oil spray
RC InnPharma Corporation
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
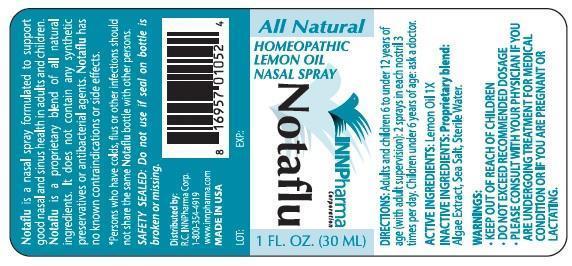
Notaflu Homeopathic Lemon Oil Nasal Spray
Notaflu is a nasal spray formulated to support good nasal and sinus health in adults and children.
Notaflu is a proprietary blend of all natural ingredients. It does not contain any synthetic preservatives or antibacterial agents. Notaflu has kno known contraindications or side effects.
*Persons who have colds, flus or other infections should not share the same Notaflu bottle with other persons.
Safety Sealed: Do not use if seal on bottle is broken or missing
Distributed by:
RC InnPharma Corp
1-800-354-4919
www.innpharma.com
MADE IN USA
DIRECTIONS
Adults and children 6 to under 12 years of age (with adult supervision): 2 sprays in each nostril 3 times per day. Children under 6 years of age ask a doctor.
| NOTAFLU LEMON OIL
lemon oil spray |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - RC InnPharma Corporation (010593096) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pure Source, LLC | 080354456 | manufacture(60698-0000) | |