LANSOPRAZOLE- lansoprazole capsule, delayed release
Rugby Laboratories, Inc.
----------
DRUG FACTS
Use
- treats frequent heartburn (occurs 2 or more days a week)
- not intended for immediate relief of heartburn; this drug may take 1 to 4 days for full effect
WARNINGS
Allergy alert: Do not use if you are allergic to lansoprazole.
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- liver disease
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Ask a doctor or pharmacist before use if you are
taking
- warfarin (blood-thinning medicine)
- prescription antifungal or anti-yeast medicines
- digoxin (heart medicine)
- theophylline (asthma medicine)
- tacrolimus (immune system medicine)
- atazanavir (medicine for HIV infection)
Directions
- adults 18 years of age and older
- this product is to be used once a day (every 24 hours), every day for 14 days
- it may take 1 to 4 days for full effect, although some people get complete relief of symptoms within 24 hours
14-Day Course of Treatment
- swallow 1 capsule with a glass of water before eating in the morning
- take every day for 14 days
- do not take more than 1 capsule a day
- swallow whole. Do not crush or chew capsules.
- do not use for more than 14 days unless directed by your doctor
Repeated 14-Day Courses (if needed)
- you may repeat a 14-day course every 4 months
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor. Patients should use the lowest dose and shortest duration of this therapy.
- children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be caused by a serious condition.
Other information
- read the directions, warnings and package insert before use
- keep the carton and package insert. They contain important information.
- store at 20°-25°C (68°-77°F)
- keep product out of high heat and humidity
- protect product from moisture
Inactive ingredients
colloidal silicon dioxide, D&C red No. 33, D&C yellow No. 10, FD&C blue No. 1, FD&C red No. 40, gelatin, hypromellose, magnesium carbonate, methacrylic acid copolymer dispersion, polyethylene glycol, polysorbate 80, sucrose, sugar spheres, talc, titanium dioxide
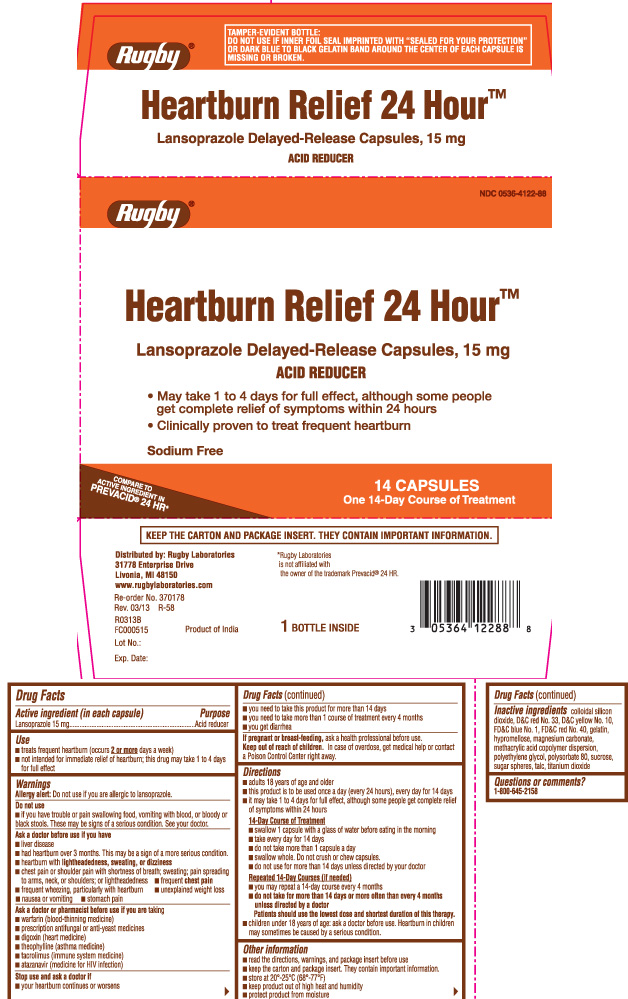
Principal Display Panel
Heartburn Relief 24 Hour™
Lansoprazole Delayed-Release Capsules, 15 mg
ACID REDUCER
- May take 1 to 4 days for full effect, although some people get complete relief of symptoms within 24 hours
- Clinically proven to treat frequent heartburn
Sodium Free
COMPARE TO ACTIVE INGREDIENT IN PREVACID® 24 HR*
CAPSULES
*Rugby Laboratories is not affiliated with the owner of the trademark Prevacid® 24 HR.
Distributed by: Rugby Laboratories
31778 Enterprise Drive
Livonia, MI 48150
Product of India
KEEP THE CARTON AND PACKAGE INSERT. THEY CONTAIN IMPORTANT INFORMATION.
TAMPER-EVIDENT BOTTLE: DO NOT USE IF INNER FOIL SEAL IMPRINTED WITH “SEALED for YOUR PROTECTION” OR DARK BLUE TO BLACK GELATIN BAND AROUND THE CENTER OF EACH CAPSULE IS MISSING OR BROKEN.
| LANSOPRAZOLE
lansoprazole capsule, delayed release |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Rugby Laboratories, Inc. (079246066) |
| Registrant - P & L Development, LLC (800014821) |