Label: STAVUDINE capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 53808-0594-1 - Packager: State of Florida DOH Central Pharmacy
- This is a repackaged label.
- Source NDC Code(s): 0378-5043
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 8, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY WITH STEATOSIS, INCLUDING FATAL CASES, HAVE BEEN REPORTED WITH THE USE OF NUCLEOSIDE ANALOGUES ALONE OR IN COMBINATION, INCLUDING STAVUDINE AND OTHER ANTIRETROVIRALS. FATAL LACTIC ACIDOSIS HAS BEEN REPORTED IN PREGNANT WOMEN WHO RECEIVED THE COMBINATION OF STAVUDINE AND DIDANOSINE WITH OTHER ANTIRETROVIRAL AGENTS. THE COMBINATION OF STAVUDINE AND DIDANOSINE SHOULD BE USED WITH CAUTION DURING PREGNANCY AND IS RECOMMENDED ONLY IF THE POTENTIAL BENEFIT CLEARLY OUTWEIGHS THE POTENTIAL RISK (SEE WARNINGS AND PRECAUTIONS: PREGNANCY)
FATAL AND NONFATAL PANCREATITIS HAVE OCCURRED DURING THERAPY WHEN STAVUDINE WAS PART OF A COMBINATION REGIMEN THAT INCLUDED DIDANOSINE, IN BOTH TREATMENT-NAIVE AND TREATMENT-EXPERIENCED PATIENTS, REGARDLESS OF DEGREE OF IMMUNOSUPPRESSION (SEE WARNINGS)
-
DESCRIPTION
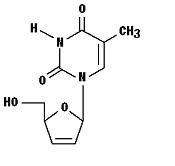
Stavudine (d4T), a synthetic thymidine nucleoside analogue, active against the human immunodeficiency virus (HIV).
Stavudine Capsules are supplied for oral administration in strengths of 15, 20, 30, and 40 mg of stavudine. Each capsule also contains inactive ingredients microcrystalline cellulose, sodium starch glycolate, lactose anhydrous, and magnesium stearate. The hard gelatin shell consists of gelatin, sodium lauryl sulfate, titanium dioxide, and iron oxides.The capsules are printed with Black ink containing black iron oxide E172 dye.
The chemical name for stavudine is 2',3'-didehydro-3'-deoxythymidine. Stavudine has the following structural formula:
Stavudine is a white to off-white crystalline solid with the molecular formula C10 H12 N2 O4 and a molecular weight of 224.2. The solubility of stavudine at 23° C is approximately 83 mg/mL in water and 30 mg/mL in propylene glycol. The n-octanol/water partition coefficient of stavudine at 23° C is 0.144.
-
MICROBIOLOGY
Mechanism of Action
Stavudine, a nucleoside analogue of thymidine, is phosphorylated by cellular kinases to the active metabolite stavudine triphosphate. Stavudine triphosphate inhibits the activity of HIV-1 reverse transcriptase (RT) by competing with the natural substrate thymidine triphosphate (Ki=0.0083 to 0.032 μM) and by causing DNA chain termination following its incorporation into viral DNA. Stavudine triphosphate inhibits cellular DNA polymerases β and γ and markedly reduces the synthesis of mitochondrial DNA.
Antiviral Activity
The cell culture antiviral activity of stavudine was measured in peripheral blood mononuclear cells, monocytic cells, and lymphoblastoid cell lines. The concentration of drug necessary to inhibit HIV-1 replication by 50% (EC50) ranged from 0.009 to 4 μM against laboratory and clinical isolates of HIV-1. In cell culture, stavudine exhibited additive to antagonistic activity in combination with zidovudine. Stavudine in combination with either abacavir, didanosine, tenofovir, or zalcitabine exhibited additive to synergistic anti-HIV-1 activity. Ribavirin, at the 9-45 μM concentrations tested, reduced the anti-HIV-1 activity of stavudine by 2.5- to 5-fold. The relationship between cell culture susceptibility of HIV-1 to stavudine and the inhibition of HIV-1 replication in humans has not been established.
Drug Resistance
HIV-1 isolates with reduced susceptibility to stavudine have been selected in cell culture (strain-specific) and were also obtained from patients treated with stavudine. Phenotypic analysis of HIV-1 isolates from 61 patients receiving prolonged (6-29 months) stavudine monotherapy showed that post-therapy isolates from four patients exhibited EC50 values more than 4-fold (range 7- to 16-fold) higher than the average pretreatment susceptibility of baseline isolates. Of these, HIV-1 isolates from one patient contained the zidovudine-resistance-associated mutations T215Y and K219E, and isolates from another patient contained the multiple-nucleoside-resistance-associated mutation Q151M. Mutations in the RT gene of HIV-1 isolates from the other two patients were not detected. The genetic basis for stavudine susceptibility changes has not been identified.
Cross-resistance
Cross-resistance among HIV-1 reverse transcriptase inhibitors has been observed. Several studies have demonstrated that prolonged stavudine treatment can select and/or maintain mutations associated with zidovudine resistance. HIV-1 isolates with one or more zidovudine-resistance-associated mutations (M41L, D67N, K70R, L210W, T215Y/F, K219Q/E) exhibited reduced susceptibility to stavudine in cell culture.
-
CLINICAL PHARMACOLOGY
Pharmacokinetics
The pharmacokinetics of stavudine have been evaluated in HIV-infected adult and pediatric patients (Tables 1-3). Peak plasma concentrations (Cmax) and area under the plasma concentration-time curve (AUC) increased in proportion to dose after both single and multiple doses ranging from 0.03 to 4 mg/kg. There was no significant accumulation of stavudine with repeated administration every 6, 8, or 12 hours.
Absorption
Following oral administration, stavudine is rapidly absorbed, with peak plasma concentrations occurring within 1 hour after dosing. Steady-state pharmacokinetic parameters of stavudine in HIV-infected adults are shown in Table 1.
Table 1: Steady-State Pharmacokinetic Parameters of Stavudine in HIV-Infected Adults Parameter Stavudine 40 mg BID Mean ± SD (n=8) AUC (ng•h/mL)a 2568 ± 454 Cmax (ng/mL) 536 ± 146 Cmin (ng/mL) 8 ± 9 afrom 0 to 24 hours
AUC = area under the curve over 24 hours.
Cmax = maximum plasma concentration.
Cmin = trough or minimum plasma concentration.Distribution
Binding of stavudine to serum proteins was negligible over the concentration range of 0.01 to 11.4µg/mL. Stavudine distributes equally between red blood cells and plasma. Volume of distribution is shown in Table 2.
Metabolism
Metabolism plays a limited role in the clearance of stavudine. Unchanged stavudine was the major drug-related component circulating in plasma after an 80-mg dose of 14C-stavudine, while metabolites constituted minor components of the circulating radioactivity. Minor metabolites include oxidized stavudine, glucuronide conjugates of stavudine and its oxidized metabolite, and an N-acetylcysteine conjugate of the ribose after glycosidic cleavage, suggesting that thymine is also a metabolite of stavudine.
Elimination
Following an 80-mg dose of 14C-stavudine to healthy subjects, approximately 95% and 3% of the total radioactivity was recovered in urine and feces, respectively. Radioactivity due to parent drug in urine and feces was 73.7% and 62.0%, respectively. The mean terminal elimination half-life is approximately 2.3 hours following single oral doses. Mean renal clearance of the parent compound is approximately 272 mL/min, accounting for approximately 67% of the apparent oral clearance
In HIV-infected patients, renal elimination of unchanged drug accounts for about 40% of the overall clearance regardless of the route of administration (Table 2). The mean renal clearance was about twice the average endogenous creatinine clearance, indicating active tubular secretion in addition to glomerular filtration.
Table 2: Pharmacokinetic Parameters of Stavudine in HIV-Infected Adults: Bioavailability, Distribution, and Clearance Parameter Mean ± SD n Oral bioavailability (%) 86.4 ±18.2 25 Volume of distribution (L)a 46 ± 21 44 Total body clearance (mL/min)a 594 ± 164 44 Apparent oral clearance (mL/min)b 560 ± 182c 113 Renal clearance (mL/min)a 237 ± 98 39 Elimination half-life, IV dose (h)a 1.15 ± 0.35 44 Elimination half-life, oral dose (h)b 1.6 ± 0.23 8 Urinary recovery of stavudine (% of dose)a,d 42 ± 14 39 a following 1-hour IV infusion.
b following single oral dose.
c assuming a body weight of 70 kg.
d over 12-24 hours.Pediatric
For pharmacokinetic properties of stavudine in pediatric patients see Table 3.
Table 3: Pharmacokinetic Parameters (Mean ±SD) of Stavudine in HIV- Exposed or -Infected Pediatric Patients
ParameterAges 5 weeks
to 15 yearsn Ages 14to
28 daysn Day of Birth n Oral bioavailability (%) 76.9 ±31.7 20 ND ND Volume of distribution (L/kg)a 0.73 ±0.32 21 ND ND Ratio of CSF: plasma concentrations (as %)b 59 ± 35 8 ND ND Total body clearance (mL/min/kg)a 9.75 ±3.76 21 ND ND Apparent oral clearance (mL/min/kg)c 13.75 ± 4.29 20 11.52 ± 5.93 30 5.08 ± 2.80 17 Elimination half-life,IV dose (h)a 1.11 ±0.28 21 ND ND Elimination half-life,oral dose (h)c 0.96 ± 0.26 20 1.59 ± 0.29 30 5.27 ± 2.01 17 Urinary recovery of stavudine (% of dose)c,d 34 ± 16 19 ND ND a following 1-hour IV infusion.
b at median time of 2.5 hours (range 2-3 hours) following multiple oral doses.
c following single oral dose.
d over 8 hours.
ND = not determined.Renal Impairment
Data from two studies in adults indicated that the apparent oral clearance of stavudine decreased and the terminal elimination half-life increased as creatinine clearance decreased (see Table 4). Cmax and Tmax were not significantly altered by renal impairment. The mean ± SD hemodialysis clearance value of stavudine was 120 ±18 mL/min (n=12); the mean ± SD percentage of the stavudine dose recovered in the dialysate, timed to occur between 2-6 hours post-dose, was 31 ± 5%. Based on these observations, it is recommended that stavudine dosage be modified in patients with reduced creatinine clearance and in patients receiving maintenance hemodialysis (see DOSAGE AND ADMINISTRATION).
Table 4: Mean ± SD Pharmacokinetic Parameter Values of Stavudinea in Adults with Varying Degrees of Renal Function Creatinine Clearance Hemodialysis
Patients b
n=11>50 mL/min
n=1026-50 mL/min
n=59-25 mL/min
n=5Creatinine clearance (mL/min) 104 ± 28 41 ± 5 17 ± 3 NA Apparent oral clearance (mL/min) 335 ± 57 191 ± 39 116 ± 25 105 ± 17 Renal clearance (mL/min) 167 ± 65 73 ± 18 17 ± 3 NA T ½ (h) 1.7 ± 0.4 3.5 ± 2.5 4.6 ± 0.9 5.4 ± 1.4
a Single 40-mg oral dose.
b Determined while patients were off dialysis.
T½ = terminal elimination half-life.
NA = not applicable.Hepatic Impairment
Stavudine pharmacokinetics were not altered in five non-HIV-infected patients with hepatic impairment secondary to cirrhosis (Child-Pugh classification B or C) following the administration of a single 40-mg dose.
Geriatric
Stavudine pharmacokinetics have not been studied in patients >65 years of age. (See PRECAUTIONS: Geriatric Use.)
Gender
A population pharmacokinetic analysis of data collected during a controlled clinical study in HIV-infected patients showed no clinically important differences between males (n=291) and females (n=27).
Race
A population pharmacokinetic analysis of data collected during a controlled clinical study in HIV-infected patients showed no clinically important differences between races (n=233 Caucasian, 39 African-American, 41 Hispanic, 1 Asian, and 4 other).
Drug Interactions
(see PRECAUTIONS: Drug Interactions)
Zidovudine: Zidovudine competitively inhibits the intracellular phosphorylation of stavudine. Therefore, use of zidovudine in combination with stavudine should be avoided.
Doxorubicin: In vitro data indicate that the phosphorylation of stavudine is inhibited at relevant concentrations by doxorubicin.
Ribavirin: In vitro data indicate ribavirin reduces phosphorylation of lamivudine, stavudine, and zidovudine. However, no pharmacokinetic (eg, plasma concentrations or intracellular triphosphorylated active metabolite concentrations) or pharmacodynamic (eg, loss of HIV/HCV virologic suppression) interaction was observed when ribavirin and lamivudine (n=18), stavudine (n=10), or zidovudine (n=6) were coadministered as part of a multi-drug regimen to HIV/HCV co-infected patients (see WARNINGS ).
Stavudine does not inhibit the major cytochrome P450 isoforms CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4; therefore, it is unlikely that clinically significant drug interactions will occur with drugs metabolized through these pathways.
Because stavudine is not protein-bound, it is not expected to affect the pharmacokinetics of protein-bound drugs.
Tables 5 and 6 summarize the effects on AUC and Cmax, with a 95% confidence interval (CI) when available, following coadministration of stavudine with didanosine, lamivudine, and nelfinavir. No clinically significant pharmacokinetic interactions were observed.
Table 5 : Results of Drug Interaction Studies with Stavudine: Effects of Coadministered Drug on Stavudine Plasma AUC and Cmax Values Drug Stavudine Dosage na AUC of Stavudine(95% CI) Cmax of Stavudine (95% CI) Didanosine, 100 mg q12h for 4 days 40 mg q12h for 4 days 10 ↔ ↑ 17% Lamivudine, 150 mg single dose 40 mg single dose 18 ↔
( 92.7 - 100.6%)↑ 12%
( 100.3 -126.1% )Nelfinavir, 750 mg q8h for 56 days 30-40 mg q12h for 56 days 8 ↔ ↔ ↑ indicates increase.
↔ indicates no change, or mean increase or decrease of <10%.
a HIV-infected patients.Table 6: Results of Drug Interaction Studies with Stavudine: Effects of Stavudine on Coadministered Drug Plasma AUC and CmaxValues Drug Stavudine Dosage na AUC of Coadministered Drug (95%CI) Cmax of Coadministered Drug (95% CI) Didanosine, 100 mg q12h for 4 days 40 mg q12h for 4 days 10 ↔ ↔ Lamivudine, 150 mg single dose 40 mg single dose 18 ↔
( 90.5 - 107.6%)↔
( 87.1 - 110.6%)Nelfinavir, 750 mg q8h for 56 days 30-40 mg q12h for 56 days 8 ↔ ↔ ↔ indicates no change, or mean increase or decrease of <10%.
a HIV-infected patients. -
INDICATIONS AND USAGE
Stavudine capsules , in combination with other antiretroviral agents, is indicated for the treatment of HIV-1 infection (see Clinical Studies).
-
Clinical Studies
Combination Therapy
The combination use of stavudine is based on the results of clinical studies in HIV-infected patients in double- and triple-combination regimens with other antiretroviral agents.
One of these studies (START 1) was a multicenter, randomized, open-label study comparing stavudine (40 mg twice daily) plus lamivudine plus indinavir to zidovudine plus lamivudine plus indinavir in 202 treatment-naive patients. Both regimens resulted in a similar magnitude of inhibition of HIV RNA levels and increases in CD4 cell counts through 48 weeks.
Monotherapy
The efficacy of stavudine was demonstrated in a randomized, double-blind study (AI455–019, conducted 1992-1994) comparing stavudine with zidovudine in 822 patients with a spectrum of HIV-related symptoms. The outcome in terms of progression of HIV disease and death was similar for both drugs.
- CONTRAINDICATIONS
-
WARNINGS
1.Lactic Acidosis / Severe Hepatomegaly with Steatosis:
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including stavudine and other antiretrovirals. Although relative rates of lactic acidosis have not been assessed in prospective well-controlled trials, longitudinal cohort and retrospective studies suggest that this infrequent event may be more often associated with antiretroviral combinations containing stavudine. Female gender, obesity, and prolonged nucleoside exposure may be risk factors. Fatal lactic acidosis has been reported in pregnant women who received the combination of stavudine and didanosine with other antiretroviral agents. The combination of stavudine and didanosine should be used with caution during pregnancy and is recommended only if the potential benefit clearly outweighs the potential risk (see PRECAUTIONS: Pregnancy ).
Particular caution should be exercised when administering stavudine to any patient with known risk factors for liver disease; however, cases of lactic acidosis have also been reported in patients with no known risk factors. Generalized fatigue, digestive symptoms (nausea, vomiting, abdominal pain, and unexplained weight loss); respiratory symptoms (tachypnea and dyspnea); or neurologic symptoms (including motor weakness, see 3. Neurologic Symptoms) might be indicative of the development of symptomatic hyperlactatemia or lactic acidosis syndrome.
Treatment with stavudine should be suspended in any patient who develops clinical or laboratory findings suggestive of symptomatic hyperlactatemia, lactic acidosis, or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
2.Hepatic Impairment and Toxicity:
The safety and efficacy of stavudine have not been established in HIV-infected patients with significant underlying liver disease. During combination antiretroviral therapy, patients with preexisting liver dysfunction, including chronic active hepatitis, have an increased frequency of liver function abnormalities, including severe and potentially fatal hepatic adverse events, and should be monitored according to standard practice. If there is evidence of worsening liver disease in such patients, interruption or discontinuation of treatment must be considered.
An increased risk of hepatotoxicity may occur in patients treated with stavudine in combination with didanosine and hydroxyurea compared to when stavudine is used alone. Deaths attributed to hepatotoxicity have occurred in patients receiving this combination. This combination should be avoided.
Use with Interferon and Ribavirin-Based Regimens
In vitro studies have shown ribavirin can reduce the phosphorylation of pyrimidine nucleoside analogues such as stavudine. Although no evidence of a pharmacokinetic or pharmacodynamic (eg, loss of HIV/HCV virologic suppression) interaction was seen when ribavirin was coadministered with stavudine in HIV/HCV co-infected patients (see CLINICAL PHARMACOLOGY: Drug Interactions), hepatic decompensation (some fatal) has occurred in HIV/HCV co-infected patients receiving combination antiretroviral therapy for HIV and interferon and ribavirin. Patients receiving interferon with or without ribavirin and stavudine should be closely monitored for treatment-associated toxicities, especially hepatic decompensation. Discontinuation of stavudine should be considered as medically appropriate. Dose reduction or discontinuation of interferon, ribavirin, or both should also be considered if worsening clinical toxicities are observed, including hepatic decompensation (eg, Child-Pugh >6) (see the complete prescribing information for interferon and ribavirin).
3. Neurologic Symptoms:
Motor weakness has been reported rarely in patients receiving combination antiretroviral therapy including stavudine. Most of these cases occurred in the setting of lactic acidosis. The evolution of motor weakness may mimic the clinical presentation of Guillain-Barré syndrome (including respiratory failure). Symptoms may continue or worsen following discontinuation of therapy.
Peripheral neuropathy, manifested by numbness, tingling, or pain in the hands or feet, has been reported in patients receiving stavudine therapy. Peripheral neuropathy has occurred more frequently in patients with advanced HIV disease, with a history of neuropathy, or in patients receiving other drugs that have been associated with neuropathy, including didanosine (see ADVERSE REACTIONS).
4. Pancreatitis
Fatal and nonfatal pancreatitis have occurred during therapy when stavudine was part of a combination regimen that included didanosine, in both treatment-naive and treatment-experienced patients, regardless of degree of immunosuppression. The combination of stavudine and didanosine and any other agents that are toxic to the pancreas should be suspended in patients with suspected pancreatitis. Reinstitution of stavudine after a confirmed diagnosis of pancreatitis should be undertaken with particular caution and close patient monitoring. The new regimen should not contain didanosine .
-
PRECAUTIONS
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including stavudine. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia (PCP), or tuberculosis), which may necessitate further evaluation and treatment.
-
Information for Patients
(See Patient Information Leaflet.)
Patients should be informed of the importance of early recognition of symptoms of symptomatic hyperlactatemia or lactic acidosis syndrome, which include unexplained weight loss, abdominal discomfort, nausea, vomiting, fatigue, dyspnea, and motor weakness. Patients in whom these symptoms develop should seek medical attention immediately. Discontinuation of stavudine therapy may be required.
Patients should be informed that an important toxicity of stavudine is peripheral neuropathy. Patients should be aware that peripheral neuropathy is manifested by numbness, tingling, or pain in hands or feet, and that these symptoms should be reported to their physicians. Patients should be counseled that peripheral neuropathy occurs with greatest frequency in patients who have advanced HIV disease or a history of peripheral neuropathy, and that dose modification and/or discontinuation of stavudine may be required if toxicity develops.
Caregivers of young children receiving stavudine therapy should be instructed regarding detection and reporting of peripheral neuropathy.
Patients should be informed that when stavudine is used in combination with other agents with similar toxicities, the incidence of adverse events may be higher than when stavudine is used alone. An increased risk of pancreatitis, which may be fatal, may occur in patients treated with the combination of stavudine and didanosine. Patients treated with this combination should be closely monitored for symptoms of pancreatitis. An increased risk of hepatotoxicity, which may be fatal, may occur in patients treated with stavudine in combination with didanosine and hydroxyurea. This combination should be avoided.
Patients should be informed that stavudine is not a cure for HIV infection, and that they may continue to acquire illnesses associated with HIV infection, including opportunistic infections. Patients should be advised to remain under the care of a physician when using stavudine. They should be advised that stavudine therapy has not been shown to reduce the risk of transmission of HIV to others through sexual contact or blood contamination. Patients should be informed that the long-term effects of stavudine are unknown at this time.
Patients should be informed that the Centers for Disease Control and Prevention (CDC) recommend that HIV-infected mothers not nurse newborn infants to reduce the risk of postnatal transmission of HIV infection.
Patients should be informed that redistribution or accumulation of body fat may occur in individuals receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known at this time.
Patients should be advised of the importance of adherence to any antiretroviral regimen, including those that contain stavudine.
-
Drug Interactions
(see also CLINICAL PHARMACOLOGY)
Zidovudine competitively inhibits the intracellular phosphorylation of stavudine. Therefore, use of zidovudine in combination with stavudine should be avoided.
In vitro data indicate that the phosphorylation of stavudine is also inhibited at relevant concentrations by doxorubicin and ribavirin. The clinical significance of these in vitro interactions is unknown; therefore, concomitant use of stavudine with either of these drugs should be undertaken with caution. (See WARNINGS.)
-
Carcinogenesis, Mutagenesis, Impairment of Fertility
In 2-year carcinogenicity studies in mice and rats, stavudine was noncarcinogenic at doses which produced exposures (AUC) 39 and 168 times, respectively, human exposure at the recommended clinical dose. Benign and malignant liver tumors in mice and rats and malignant urinary bladder tumors in male rats occurred at levels of exposure 250 (mice) and 732 (rats) times human exposure at the recommended clinical dose.
Stavudine was not mutagenic in the Ames, E.coli reverse mutation, or the CHO/HGPRT mammalian cell forward gene mutation assays, with and without metabolic activation. Stavudine produced positive results in the in vitro human lymphocyte clastogenesis and mouse fibroblast assays, and in the in vivo mouse micronucleus test. In the in vitro assays, stavudine elevated the frequency of chromosome aberrations in human lymphocytes (concentrations of 25 to 250 μg/mL, without metabolic activation) and increased the frequency of transformed foci in mouse fibroblast cells (concentrations of 25 to 2500 μg/mL, with and without metabolic activation). In the in vivo micro-nucleus assay, stavudine was clastogenic in bone marrow cells following oral stavudine administration to mice at dosages of 600 to 2000 mg/kg/day for 3 days.
No evidence of impaired fertility was seen in rats with exposures (based on Cmax ) up to 216 times that observed following a clinical dosage of 1 mg/kg/day
-
PREGNANCY
Pregnancy Category C
Reproduction studies have been performed in rats and rabbits with exposures (based on Cmax) up to 399 and 183 times, respectively, of that seen at a clinical dosage of 1 mg/kg/day and have revealed no evidence of teratogenicity. The incidence in fetuses of a common skeletal variation, unossified or incomplete ossification of sternebra, was increased in rats at 399 times human exposure, while no effect was observed at 216 times human exposure. A slight post-implantation loss was noted at 216 times the human exposure with no effect noted at approximately 135 times the human exposure. An increase in early rat neonatal mortality (birth to 4 days of age) occurred at 399 times the human exposure, while survival of neonates was unaffected at approximately 135 times the human exposure. A study in rats showed that stavudine is transferred to the fetus through the placenta. The concentration in fetal tissue was approximately one-half the concentration in maternal plasma. Animal reproduction studies are not always predictive of human response.
There are no adequate and well-controlled studies of stavudine in pregnant women. Stavudine should be used during pregnancy only if the potential benefit justifies the potential risk.
Fatal lactic acidosis has been reported in pregnant women who received the combination of stavudine and didanosine with other antiretroviral agents. It is unclear if pregnancy augments the risk of lactic acidosis/hepatic steatosis syndrome reported in nonpregnant individuals receiving nucleoside analogues (see WARNINGS: Lactic Acidosis/Severe Hepatomegaly with Steatosis). The combination of stavudine and didanosine should be used with caution during pregnancy and is recommended only if the potential benefit clearly outweighs the potential risk. Healthcare providers caring for HIV-infected pregnant women receiving stavudine should be alert for early diagnosis of lactic acidosis/hepatic steatosis syndrome.
-
Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breast-feed their infants to avoid risking postnatal transmission of HIV. Studies in lactating rats demonstrated that stavudine is excreted in milk. Although it is not known whether stavudine is excreted in human milk, there exists the potential for adverse effects from stavudine in nursing infants. Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breast-feed if they are receiving stavudine.
-
Pediatric Use
Use of stavudine in pediatric patients from birth through adolescence is supported by evidence from adequate and well-controlled studies of stavudine in adults with additional pharmacokinetic and safety data in pediatric patients.
Adverse events and laboratory abnormalities reported to occur in pediatric patients in clinical studies were generally consistent with the safety profile of stavudine in adults. These studies include ACTG 240, where 105 pediatric patients ages 3 months to 6 years received stavudine 2 mg/kg/day for a median of 6.4 months; a controlled clinical trial where 185 newborns received stavudine 2 mg/kg/day either alone or in combination with didanosine from birth through 6 weeks of age; and a clinical trial where 8 newborns received stavudine 2 mg/kg/day in combination with didanosine and nelfinavir from birth through 4 weeks of age.
Stavudine pharmacokinetics have been evaluated in 25 HIV-infected pediatric patients ranging in age from 5 weeks to 15 years and in weight from 2 to 43 kg after IV or oral administration of single doses and twice-daily regimens and in 30 HIV-exposed or -infected newborns ranging in age from birth to 4 weeks after oral administration of twice-daily regimens (see CLINICAL PHARMACOLOGY, Table 3).
-
Geriatric Use
Clinical studies of stavudine did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently than younger patients. Greater sensitivity of some older individuals to the effects of stavudine cannot be ruled out.
In a monotherapy Expanded Access Program for patients with advanced HIV infection, peripheral neuropathy or peripheral neuropathic symptoms were observed in 15 of 40 (38%) elderly patients receiving 40 mg twice daily and 8 of 51 (16%) elderly patients receiving 20 mg twice daily. Of the approximately 12,000 patients enrolled in the Expanded Access Program, peripheral neuropathy or peripheral neuropathic symptoms developed in 30% of patients receiving 40 mg twice daily and 25% of patients receiving 20 mg twice daily. Elderly patients should be closely monitored for signs and symptoms of peripheral neuropathy.
Stavudine is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function. Dose adjustment is recommended for patients with renal impairment (see DOSAGE AND ADMINISTRATION: Dosage Adjustment).
-
ADVERSE REACTIONS
Adults
Fatal lactic acidosis has occurred in patients treated with stavudine in combination with other antiretroviral agents. Patients with suspected lactic acidosis should immediately suspend therapy with stavudine . Permanent discontinuation of stavudine should be considered for patients with confirmed lactic acidosis.
Stavudine therapy has rarely been associated with motor weakness, occurring predominantly in the setting of lactic acidosis. If motor weakness develops, stavudine should be discontinued.
Stavudine therapy has also been associated with peripheral sensory neuropathy, which can be severe, is dose related, and occurs more frequently in patients being treated with other drugs that have been associated with neuropathy (including didanosine), in patients with advanced HIV infection, or in patients who have previously experienced peripheral neuropathy.
Patients should be monitored for the development of neuropathy, which is usually manifested by numbness, tingling, or pain in the feet or hands. Stavudine-related peripheral neuropathy may resolve if therapy is withdrawn promptly. In some cases, symptoms may worsen temporarily following discontinuation of therapy. If symptoms resolve completely, patients may tolerate resumption of treatment at one-half the dose (see DOSAGE AND ADMINISTRATION). If neuropathy recurs after resumption, permanent discontinuation of stavudine should be considered.
Selected clinical adverse events that occurred in adult patients receiving stavudine in a controlled monotherapy study (Study AI455-019) are provided in Table 7.
Table 7: Selected Clinical Adverse Events in study AI455-019a (Monotherapy) Percent (%) Adverse Events Stavudineb
( 40 mg twice daily)
(n=412)Zidovudine
(200 mg 3 times daily)
(n=402)Headache 54 49 Diarrhea 50 44 Peripheral Neurologic
Symptoms/Neuropathy52 39 Rash 40 35 Nausea and Vomiting 39 44 a Any severity, regardless of relationship to study drug.
b Median duration of stavudine therapy = 79 weeks; median duration of zidovudine therapy = 53 weeks.Pancreatitis was observed in 3 of the 412 adult patients who received stavudine in a controlled monotherapy study.
Selected clinical adverse events that occurred in antiretroviral- naive adult patients receiving stavudine from two controlled combination studies are provided in Table 8.
Table 8 : Selected Clinical Adverse Eventsa in START 1 and START 2b studies (Combination Therapy) Percent (%)
START 1 START 2b Adverse Events Stavudine + lamivudine + indinavir (n=100c) zidovudine + lamivudine + indinavir (n=102) Stavudine + didanosine + indinavir (n=102c) zidovudine + lamivudine + indinavir (n=103) Nausea 43 63 53 67 Diarrhea 34 16 45 39 Headache 25 26 46 37 Rash 18 13 30 18 Vomiting 18 33 30 35 Peripheral Neurologic Symptoms/Neuropathy 8 7 21 10 a Any severity, regardless of relationship to study regimen.
b START 2 compared two triple-combination regimens in 205 treatment-naive patients. Patients received either stavudine(40 mg twice daily) plus didanosine plus indinavir or zidovudine plus lamivudine plus indinavir.
c Duration of stavudine therapy = 48 weeks.Pancreatitis resulting in death was observed in patients treated with stavudine plus didanosine, in controlled clinical studies and in postmarketing reports.
Selected laboratory abnormalities reported in a controlled monotherapy study (Study AI455-019) are provided in Table 9.
Table 9: Selected Adult Laboratory Abnormalities in Study AI455-019a,b Percent (%) Parameter Stavudine
(40 mg twice daily) (n=412)Zidovudine
(200 mg 3 times daily) (n=402)AST (SGOT) (>5.0 x ULN) 11 10 ALT (SGPT) (>5.0 x ULN) 13 11 Amylase ( >1.4 x ULN) 14 13 a Data presented for patients for whom laboratory evaluations were performed. b Median duration of stavudine therapy = 79 weeks; median duration of zidovudine therapy = 53 weeks. ULN = upper limit of normal. Selected laboratory abnormalities reported in two controlled combination studies are provided in Tables 10 and 11.
Table 10: Selected Laboratory Abnormalities in START 1 and START 2 Studies (Grades 3-4) Percent (%) START 1 START 2 Parameter
Stavudine + lamivudine+ indinavir
(n=100)Zidovudine + lamivudine + indinavir
(n=102)Stavudine + didanosine + indinavir
(n=102)zidovudine + lamivudine + indinavir
(n=103)Bilirubin (>2.6 x ULN) 7 6 16 8 AST (SGOT) (>5 x ULN) 5 2 7 7 ALT (SGPT) (>5 x ULN) 6 2 8 5 GGT (>5 x ULN) 2 2 5 2 Lipase (>2 x ULN) 6 3 5 5 Amylase (>2 x ULN) 4 <1 8 2 ULN = upper limit of normal. Table 11: Selected Laboratory Abnormalities in START 1 and START 2 Studies (All Grades) Percent (%) START 1 START 2 Parameter
Stavudine + lamivudine
+ indinavir
(n=100)Zidovudine + lamivudine
+ indinavir
(n=102)Stavudine + didanosine +
indinavir
(n=102)zidovudine + lamivudine +
indinavir
(n=103)Total Bilirubin 65 60 68 55 AST (SGOT) 42 20 53 20 ALT (SGPT) 40 20 50 18 GGT 15 8 28 12 Lipase 27 12 26 19 Amylase 21 19 31 17 Observed During Clinical Practice
The following events have been identified during post-approval use of stavudine. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to their seriousness, frequency of reporting, causal connection to stavudine, or a combination of these factors.
Body as a Whole- abdominal pain, allergic reaction, chills/fever, and redistribution/accumulation of body fat (see PRECAUTIONS: Fat Redistribution).
Digestive Disorders- anorexia.
Exocrine Gland Disorders- pancreatitis [including fatal cases (see WARNINGS)].
Hematologic Disorders- anemia, leukopenia, thrombocytopenia, and macrocytosis.
Liver- symptomatic hyperlactatemia/lactic acidosis and hepatic steatosis (see WARNINGS), hepatitis and liver failure.
Metabolic Disorders—diabetes mellitus and hyperglycemia.
Musculoskeletal- myalgia.
Nervous System - insomnia, severe motor weakness (most often reported in the setting of lactic acidosis, see WARNINGS).
Use with Didanosine- and Hydroxyurea-Based Regimens
When stavudine is used in combination with other agents with similar toxicities, the incidence of these toxicities may be higher than when stavudine used alone. Thus, patients treated with stavudine in combination with didanosine, with or without hydroxyurea, may be at increased risk for pancreatitis and hepatotoxicity, which may be fatal, and severe peripheral neuropathy. The combination of stavudine and hydroxyurea, with or without didanosine, should be avoided (see WARNINGS and PRECAUTIONS).
Pediatric Patients
Adverse reactions and serious laboratory abnormalities in pediatric patients from birth through adolescence were similar in type and frequency to those seen in adult patients (see PRECAUTIONS: Pediatric Use).
-
OVERDOSAGE
Experience with adults treated with 12 to 24 times the recommended daily dosage revealed no acute toxicity. Complications of chronic overdosage include peripheral neuropathy and hepatic toxicity. Stavudine can be removed by hemodialysis; the mean ±SD hemodialysis clearance of stavudine is 120 ± 18 mL/min. Whether stavudine is eliminated by peritoneal dialysis has not been studied.
-
DOSAGE AND ADMINISTRATION
The interval between doses of stavudine should be 12 hours. Stavudine may be taken with or without food.
Adults:The recommended dose based on body weight is as follows:
40 mg twice daily for patients ≥ 60 kg.
30 mg twice daily for patients < 60 kg.
Pediatrics: The recommended dose for newborns from birth to 13 days old is 0.5 mg/kg/dose given every 12 hours (see CLINICAL PHARMACOLOGY). The recommended dose for pediatric patients at least 14 days old and weighing less than 30 kg is 1 mg/kg/dose, given every 12 hours. Pediatric patients weighing 30 kg or greater should receive the recommended adult dosage.
Dosage Adjustment
Patients should be monitored for the development of peripheral neuropathy, which is usually manifested by numbness, tingling, or pain in the feet or hands. These symptoms may be difficult to detect in young children (see WARNINGS). If these symptoms develop during treatment, stavudine therapy should be interrupted. Symptoms may resolve if therapy is withdrawn promptly. In some cases, symptoms may worsen temporarily following discontinuation of therapy. If symptoms resolve completely, patients may tolerate resumption of treatment at one-half the recommended dose:
20 mg twice daily for patients ≥ 60 kg.
15 mg twice daily for patients < 60 kg.
If peripheral neuropathy recurs after resumption of stavudine, permanent discontinuation should be considered.
Renal Impairment
Stavudine may be administered to adult patients with impaired renal function with adjustment in dose as shown in Table 12.
Table 12: Recommended Dosage Adjustment for Renal Impairment Creatinine Clearance
Recommended Stavudine Dose by Patient Weight (mL/min) ≥60 kg <60 kg >50 40 mg every 12 hours 30 mg every 12 hours 26-50 20 mg every 12 hours 15 mg every 12 hours 10-25 20 mg every 24 hours 15 mg every 24 hours Since urinary excretion is also a major route of elimination of stavudine in pediatric patients, the clearance of stavudine may be altered in children with renal impairment. Although there are insufficient data to recommend a specific dose adjustment of stavudine in this patient population, a reduction in the dose and/or an increase in the interval between doses should be considered.
- HOW SUPPLIED
- Storage:
-
PATIENT INFORMATION
Rx only
Stavudine Capsules
What is stavudine?
Stavudine is a prescription medicine used in combination with other drugs to treat adults and children who are infected with HIV (the human immunodeficiency virus), the virus that causes AIDS. Stavudine belongs to a class of drugs called nucleoside reverse transcriptase inhibitors (NRTIs). By reducing the growth of HIV, stavudine helps your body maintain its supply of CD4 cells, which are important for fighting HIV and other infections.
Stavudine will not cure your HIV infection. At present there is no cure for HIV infection. Even while taking stavudine, you may continue to have HIV-related illnesses, including infections caused by other disease-producing organisms. Continue to see your doctor regularly and report any medical problems that occur.
Stavudine does not prevent a person infected with HIV from passing the virus to other people. To protect others, you must continue to practice safe sex and take precautions to prevent others from coming in contact with your blood and other body fluids.
There is limited information on the long-term use of stavudine.
Who should not take stavudine?
Do not take stavudine if you are allergic to any of its ingredients, including its active ingredient, stavudine, and the inactive ingredients. (See Inactive Ingredients at the end of this leaflet.) Tell your doctor if you think you have had an allergic reaction to any of these ingredients.
How should I take stavudine? How should I store it?
Your doctor will determine your dose (the amount in each capsule) based on your body weight, kidney and liver function, and any side effects that you may have had with other medicines. Take stavudine exactly as instructed. Try not to miss a dose, but if you do, take it as soon as possible. If it is almost time for the next dose, skip the missed dose and continue your regular dosing schedule. Stavudine may be taken with food or on an empty stomach.
Stavudine capsules are usually taken twice a day (every 12 hours). Store stavudine capsules in a tightly closed container at room temperature away from heat and out of the reach of children and pets. Do NOT store this medicine in a damp place such as a bathroom medicine cabinet or near the kitchen sink.
If you have a kidney problem: If your kidneys are not working properly, your doctor may monitor your kidney function while you take stavudine. Also, your dosage of stavudine may be adjusted.
What should I do if someone takes an overdosage of stavudine?
If you suspect that you or someone else has taken an overdose of stavudine, get medical help right away. Contact a doctor or a poison control center.
What important information should I know about taking stavudine with other medicines?
- Do not take zidovudine (AZT) while taking stavudine, because AZT may interfere with the actions of stavudine. Products containing AZT include Combivir® ,Retrovir®, and Trizivir®.
- If you are taking ribavirin or interferon, your doctor may need to monitor your therapy more closely or may consider a change in your therapy.
Tell your doctor or pharmacist about any other medicine, vitamin, supplement, or herbal preparation you are taking.
What about pregnancy and nursing (breast-feeding)?
- It is not known if stavudine can harm a human fetus. Pregnant women have experienced serious side effects when taking stavudine (the active ingredient in stavudine) in combination with didanosine and other HIV medicines. Stavudine should be used during pregnancy only after discussion with your doctor. Tell your doctor if you become pregnant or plan to become pregnant while taking stavudine.
- Because studies have shown stavudine is in the breast milk of animals receiving the drug, it may be present in human breast milk. The Centers for Disease Control and Prevention (CDC) recommends that HIV-infected mothers not breast-feed to reduce the risk of passing HIV infection to their babies and the potential for serious adverse reactions in nursing infants. Therefore, do not nurse a baby while taking stavudine.
What are the possible side effects of stavudine?
- Lactic acidosis, severe increase of lactic acid in the blood, severe liver enlargement, including inflammation (pain and swelling) of the liver, and liver failure, which can cause death, have been reported among patients taking stavudine. Symptoms of lactic acidosis may include:
· nausea, vomiting, or unusual or unexpected stomach discomfort;
· feeling very weak and tired;
· shortness of breath;
· weakness in arms and legs.
If you notice these symptoms or if your medical condition has suddenly changed, stop taking stavudine and call your doctor right away. Lactic acidosis is a medical emergency that must be treated in a hospital. Women (including pregnant women), overweight patients, and those who have had lengthy treatment with nucleoside medicines are more likely to develop lactic acidosis. Your doctor should closely monitor your liver function if you are taking stavudine and have a history of heavy alcohol use or a liver condition.
- Peripheral neuropathy is a nerve disorder of the hands and feet. If not recognized promptly, this disorder may worsen. Tell your doctor right away if you or a child taking stavudine has continuing numbness, tingling, burning, or pain in the feet and/or hands.A child may not recognize these symptoms or know to tell you that his or her feet or hands are numb, burning, tingling, or painful. Ask your child's doctor for instructions on how to find out if your child develops peripheral neuropathy
Let your doctor know if you or a child taking stavudine has ever had peripheral neuropathy, because this condition occurs more often in patients who have had it previously. Peripheral neuropathy is also more likely to occur in patients taking drugs that affect the nerves and in patients with advanced HIV disease, but it can occur at any disease stage. If you develop peripheral neuropathy, your doctor may tell you to stop taking stavudine. In some cases the symptoms worsen for a short time before getting better. Once symptoms of peripheral neuropathy go away completely, stavudine may be started again at a lower dose.
- Pancreatitis is a dangerous inflammation of the pancreas. It may cause death. Tell your doctor right away if you develop stomach pain, nausea, or vomiting. These can be signs of pancreatitis. Let your doctor know if you have ever had pancreatitis, regularly drink alcoholic beverages, or have gallstones. Pancreatitis occurs more often in patients with these conditions. It is also more likely in people with advanced HIV disease, but can occur at any disease stage. The combination of stavudine and didanosine, may increase your risk for pancreatitis.
People who take stavudine along with other medicines that may cause similar side effects may have a higher chance of developing these side effects than if they took stavudine alone.
Other side effects. In addition to peripheral neuropathy, the most frequent side effects observed in studies of adults taking the recommended dose of stavudine were headache, diarrhea, rash, nausea, and vomiting. Other side effects may include abdominal pain, muscle pain, insomnia, loss of appetite, chills or fever, allergic reactions, blood disorders, and high blood sugar (hyperglycemia or diabetes).
Changes in body fat have been seen in some patients taking antiretroviral therapy. These changes may include increased amount of fat in the upper back and neck (“buffalo hump”), breast, and around the trunk. Loss of fat from the legs, arms, and face may also happen. The cause and long-term health effects of these conditions are not known at this time.
What else should I know about stavudine?
Inactive Ingredients:
Stavudine Capsules: microcrystalline cellulose, sodium starch glycolate, lactose anhydrous, and magnesium stearate in a hard gelatin shell. The hard gelatin shell consists of gelatin, sodium lauryl sulfate, titanium dioxide, and iron oxides.
The capsules are printed with Black ink containing black iron oxide E172 dye.
This medicine was prescribed for your particular condition. Do not use stavudine for another condition or give it to others. Keep stavudine and all other medicines out of the reach of children and pets at all times. Do not keep medicine that is out of date or that you no longer need. Dispose of unused stavudine through community take-back disposal programs when available or by placing it in an unrecognizable closed container in the household trash.
This summary does not include everything there is to know about stavudine. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. If you have questions or concerns, or want more information about stavudine, your physician and pharmacist have the complete prescribing information upon which this leaflet was based. You may want to read it and discuss it with your doctor or other healthcare professional. Remember, no written summary can replace careful discussion with your doctor.
Combivir®, Retrovir®, and Trizivir®are registered trademarks of the GlaxoSmithKline group of companies and not the trademarks of Hetero Drugs Limited.
Manufactured By:
Mylan Pharmaceuticals Inc
Morgantown, WV
USAThis Product was Repackaged By:
State of Florida DOH Central Pharmacy
104-2 Hamilton Park Drive
Tallahassee, FL 32304
United States
- Label Image 40mg
-
INGREDIENTS AND APPEARANCE
STAVUDINE
stavudine capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53808-0594(NDC:0378-5043) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STAVUDINE (UNII: BO9LE4QFZF) (STAVUDINE - UNII:BO9LE4QFZF) STAVUDINE 40 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) GELATIN (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color ORANGE Score no score Shape CAPSULE Size 1mm Flavor Imprint Code M138 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53808-0594-1 30 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079069 07/01/2009 Labeler - State of Florida DOH Central Pharmacy (829348114) Establishment Name Address ID/FEI Business Operations State of Florida DOH Central Pharmacy 829348114 repack