SENNA-LAX- sennosides tablet
L&R Distributors, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Select Brand 44-298-delisted
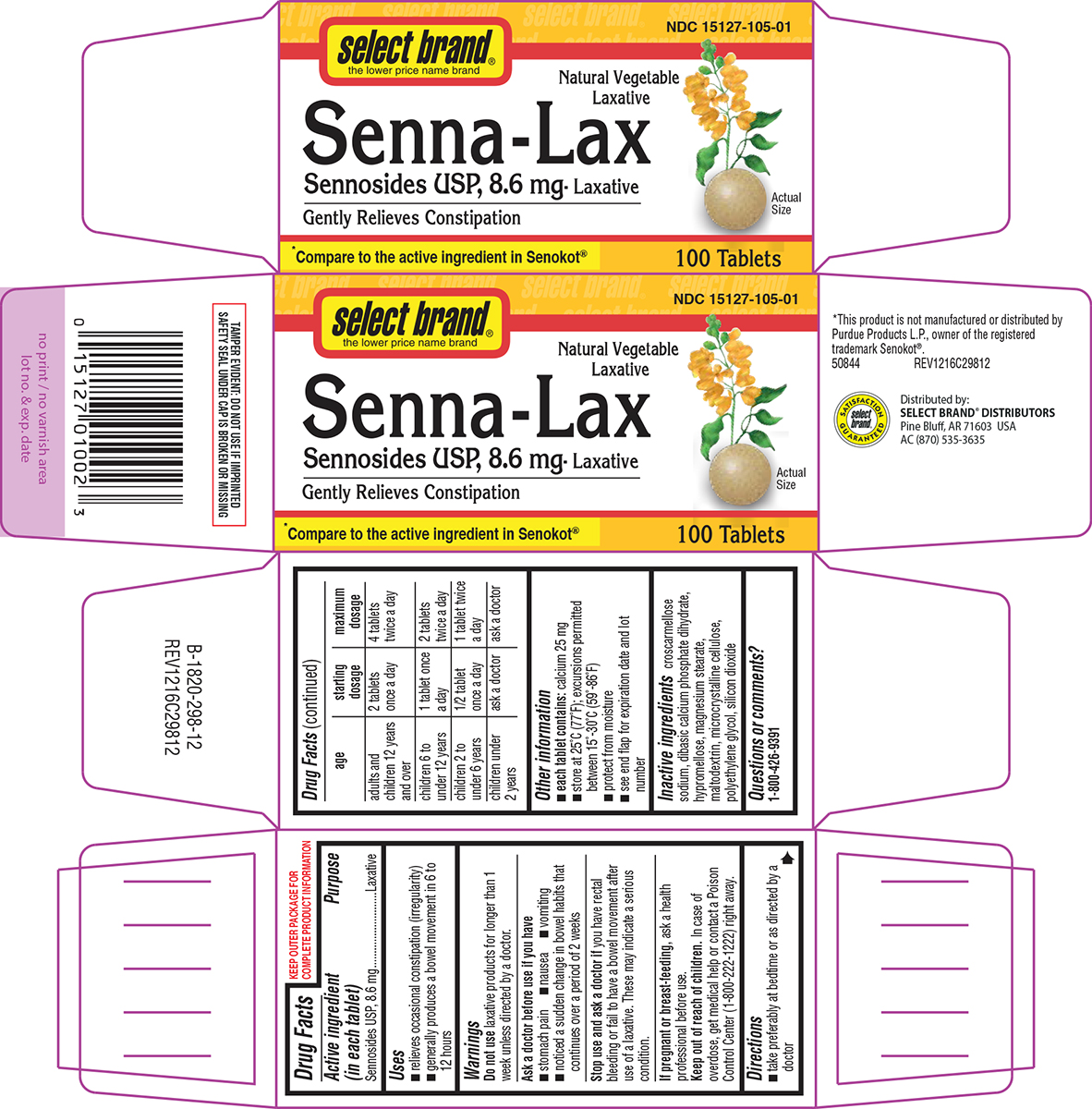
Uses
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 6 to 12 hours
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that continues over a period of 2 weeks
Directions
- take preferably at bedtime or as directed by a doctor
| age | starting dosage | maximum dosage |
| adults and children 12 years and over | 2 tablets once a day | 4 tablets twice a day |
| children 6 to under 12 years | 1 tablet once a day | 2 tablets twice a day |
| children 2 to under 6 years | 1/2 tablet once a day | 1 tablet twice a day |
| children under 2 years | ask a doctor | ask a doctor |
Other information
- each tablet contains: calcium 25 mg
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- protect from moisture
- see end flap for expiration date and lot number
Inactive ingredients
croscarmellose sodium, dibasic calcium phosphate dihydrate, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, silicon dioxide
Principal Display Panel
select brand®
the lower price name brand
NDC 15127-105-01
Natural Vegetable
Laxative
Senna-Lax
Sennosides USP, 8.6 mg- Laxative
Gently Relieves Constipation
Actual
Size
*Compare to the active ingredient in Senokot®
100 Tablets
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by
Purdue Products L.P., owner of the registered
trademark Senokot®.
50844 REV1216C29812
Distributed by:
SELECT BRAND® DISTRIBUTORS
Pine Bluff, AR 71603 USA
AC (870) 535-3635
Select Brand 44-298
| SENNA-LAX
sennosides tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - L&R Distributors, Inc. (012578514) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | MANUFACTURE(15127-105) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | PACK(15127-105) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | PACK(15127-105) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | PACK(15127-105) | |