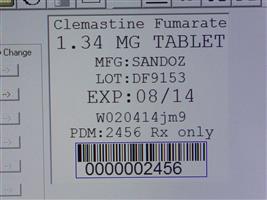
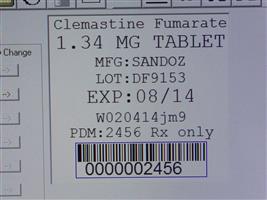
Label: CLEMASTINE FUMARATE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 68151-2456-0 - Packager: Carilion Materials Management
- This is a repackaged label.
- Source NDC Code(s): 0781-1358
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 9, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Keep out of reach of children.
- Uses
-
Warnings
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- Directions
- Other Information
- Inactive Ingredients
- Clemastine Fumarate 1.34 MG TAB
-
INGREDIENTS AND APPEARANCE
CLEMASTINE FUMARATE
clemastine fumarate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68151-2456(NDC:0781-1358) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLEMASTINE FUMARATE (UNII: 19259EGQ3D) (CLEMASTINE - UNII:95QN29S1ID) CLEMASTINE FUMARATE 1.34 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE Score 2 pieces Shape CAPSULE (capsule shaped) Size 4mm Flavor Imprint Code GG;159 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68151-2456-0 1 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA073458 10/31/1993 Labeler - Carilion Materials Management (079239644) Registrant - Carilion Materials Management (079239644) Establishment Name Address ID/FEI Business Operations Carilion Materials Management 079239644 REPACK(68151-2456)