MANNITOL- mannitol injection, solution
American Regent, Inc.
----------
MANNITOL
INJECTION, USP
25% CONCENTRATION
DESCRIPTION
Mannitol is an osmotic diuretic. It is a 6-carbon sugar alcohol with a molecular weight of 182.17. Its molecular formula is C6H14O6 and its structural formula is:
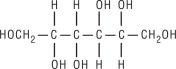
Mannitol occurs naturally in fruits and vegetables. Mannitol Injection, USP is a sterile, nonpyrogenic, 25 percent solution of Mannitol in Water for Injection. It is a supersaturated solution at room temperature.
Each 50 mL contains: Mannitol 12.5 g, Water for Injection q.s. pH adjusted with Hydrochloric Acid and/or Sodium Hydroxide. The calculated osmolarity is 1.373 milliosmols per milliliter.
CLINICAL PHARMACOLOGY
After intravenous injection, Mannitol is confined to the extracellular space, metabolized only slightly and excreted rapidly by the kidneys. Approximately 80 percent of a 100 g dose appears in the urine in 3 hours. Mannitol is freely filtered by the glomeruli with less than 10 percent tubular reabsorption. It is not secreted by tubular cells. It induces diuresis by elevating the osmolarity of the glomerular filtrate and thereby hinders tubular reabsorption of water. Urinary output of water and excretion of sodium and chloride are enhanced. Mannitol is poorly absorbed from the gastrointestinal tract. Mannitol solution is free of electrolytes and is used in urology as a nonhemolytic irrigant. The amount of mannitol absorbed intravascularly during transurethral prostatic surgery is variable and depends primarily on the extent of the surgery. Such mannitol is excreted by the kidneys and produces osmotic diuresis.
INDICATIONS AND USAGE
For Intravenous Injection: Mannitol Injection, USP is indicated for:
- Promotion of diuresis, in the prevention and/or treatment of the oliguric phase of acute renal failure before irreversible renal failure becomes established.
- Reduction of intracranial pressure and treatment of cerebral edema by reducing brain mass.
- Reduction of elevated intraocular pressure when it cannot be lowered by other means.
- Promotion of urinary excretion of toxic substances.
CONTRAINDICATIONS
Well-established anuria due to severe renal disease. Severe pulmonary congestion or frank pulmonary edema. Active intracranial bleeding except during craniotomy. Severe dehydration. Progressive renal damage or dysfunction after institution of mannitol therapy, including increasing oliguria and azotemia.
Progressive heart failure or pulmonary congestion after institution of mannitol therapy.
WARNINGS
In severe impairment of renal function a test dose should be given (see DOSAGE AND ADMINISTRATION). A second test dose may be given if there is an adequate response. No more than two test doses should be attempted.
Excessive loss of water and electrolytes may lead to serious imbalances.
Serum sodium and potassium should be carefully monitored during mannitol therapy.
The diuresis after rapid infusion of mannitol may increase preexisting hemoconcentration. With continued use of mannitol a loss of water in excess of electrolytes can cause hypernatremia.
Shift of sodium-free intracellular fluid into the extracellular compartment after mannitol infusion may lower serum sodium concentration and aggravate pre-existing hyponatremia.
Closely monitor the urine output and discontinue mannitol infusion promptly if output is low. Inadequate urine output results in accumulation of mannitol, expansion of extracellular fluid volume and could result in water intoxication or congestive heart failure. Renal function must be closely monitored during mannitol infusion.
Mannitol solution must be used with caution in patients with significant cardiopulmonary or renal dysfunction. Irrigating solutions used in transurethral prostatectomy have been shown to enter the systemic circulation in relatively large volumes, exert a systemic effect and may significantly alter cardiopulmonary and renal dynamics.
PRECAUTIONS
General: If crystals are present in mannitol, 25 percent solution, dissolve only by one of two methods: 1. Heat water bath to 80°C. Remove it from heat source then immerse vial (15-20 minutes), or, 2. Autoclave for 20 minutes at 120°C (15 psi). As soon as cool enough to handle, shake gently several times. The heat treatment can be repeated as often as necessary if the crystals have not completely dissolved or have reformed. Allow to cool to body temperature before use. Do not use vial if crystals are present.
NOTE: Use of other methods to heat the vial may result in its explosion.
The cardiovascular status should be carefully evaluated before use of mannitol rapidly intravenously or before and during transurethral resection since expansion of extracellular fluid may lead to fulminating congestive heart failure.
By sustaining diuresis, mannitol may obscure and intensify inadequate hydration or hypovolemia.
Unless it is essential, electrolyte-free mannitol solutions should not be combined with blood. When it is essential to give the combination, at least 20 mEq of sodium chloride should be added to each liter of mannitol solution to avoid pseudoagglutination.
Carcinogenesis, Mutagenesis, Impairment of Fertility: In an early study of 1, 5 or 10 percent mannitol, given for 94 weeks in the diet of Wistar rats, a low incidence of benign thymomas occurred in females which was apparently treatment related. A subsequent life-time study at similar dose levels in Spraque-Dawley, Fischer, and Wistar rats revealed no carcinogenic effect in the thymus.
Mannitol had no mutagenic activity in a series of in vitro and in vivo test systems.
Adequate studies measuring the effects of mannitol on fertility have not been done.
Pregnancy: Pregnancy Category B: Teratogenic studies in the mouse, rat, and rabbit at oral doses up to 1600 mg/kg did not reveal harm to the fetus or adverse effects on reproduction due to mannitol. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
ADVERSE REACTIONS
Reactions are infrequent and may include:
Metabolic: fluid and electrolyte imbalance, acidosis, dehydration.
Gastrointestinal: dryness of mouth, nausea, vomiting, diarrhea.
Genitourinary: osmotic nephrosis, urinary retention.
Central Nervous System: headache, convulsions, dizziness.
Special Senses: Blurred vision, rhinitis.
Cardiovascular: pulmonary edema, hypotension, hypertension, tachycardia, angina-like chest pains.
Dermatologic: skin necrosis, thrombophlebitis.
Hypersensitivity: urticaria.
Miscellaneous: thirst, arm pain, chills, fever.
DOSAGE AND ADMINISTRATION
Inspect the vial visually for particulate matter and discoloration prior to use. The contents of opened containers should be used promptly and unused contents should be discarded.
For Intravenous Injection: General Recommendations – Give mannitol injection only intravenously. The total dosage, concentration and rate of administration should be governed by the nature and severity of the condition being treated, fluid requirement and urinary output.
Usual adult dosage ranges from 50 to 200 g in 24 hours but in most instances an adequate response will be achieved at a dosage of approximately 100 g in 24 hours. The rate is usually adjusted to maintain an adequate urine flow (at least 30 to 50 mL an hour).
Test Dose – In marked oliguria or inadequate renal function a test dose of mannitol should be given. The test dose may be approximately 0.2 g/kg (about 50 mL of a 25% solution) infused in 3 to 5 minutes to produce an adequate urine flow (at least 30 to 50 mL/hr). If urine flow does not increase within 2 or 3 hours a second test dose may be given. If there is an inadequate response the patient should be re-evaluated.
Prevention of Acute Renal Failure (Oliguria): When used during surgery, immediately postoperatively or following trauma, 50 to 100 g of mannitol as a 5 to 25 percent solution may be given. The concentration and amount will depend upon the fluid requirements of the patient. Following suspected or actual hemolytic transfusion reactions 20 g of mannitol may be given intravenously over a 5 minute period to provoke diuresis. If diuresis does not occur the 20 g dose may be repeated. If there is an adequate urine flow (30 to 50 mL an hour) then intravenous fluids containing not more than 50 to 75 mEq of sodium per liter should be given in sufficient volume to match the desired urine flow (100 mL/hr) until fluids can be taken orally.
Treatment of Oliguria: The usual dose for treatment of oliguria is 50 to 100 g as a 15 to 25 percent solution.
Reduction of intracranial pressure, cerebral edema or intraocular pressure: A 25 percent solution of mannitol is recommended since its effectiveness depends on establishing intravascular hyperosmolarity. When used before or after surgery, a total dose of 1.5 to 2 g/kg can be given over a period of 30 to 60 minutes. Careful evaluation must be made of the circulatory and renal reserve prior to and during use of mannitol at this relatively high dose and rapid infusion rate. Careful attention must be paid to fluid and electrolyte balance, body weight, and total input and output before and after infusion of mannitol. Evidence of reduced cerebral spinal fluid pressure may be observed within 15 minutes after starting infusion.
Maximal reduction of intraocular pressure occurs 30 to 60 minutes after injection.
Urinary excretion of toxic substances: Mannitol in 5 to 25 percent solutions is used as an infusion as long as indicated if the level of urinary output remains high. The concentration will depend upon the fluid requirement and urinary output. Intravenous water and electrolytes must be given to replace the loss of these substances in the urine, sweat and expired air. If benefits are not observed after 200 g of mannitol are given discontinue it.
PREPARATION OF SOLUTION for intravenous injection:
Test dose – As supplied (25%)
| 5% = 50 mL of mannitol plus 200 mL | of Dextrose 5% Injection or appropriate electrolyte vehicle |
| 10% = 50 mL of mannitol plus 75 mL | |
| 15% = 50 mL of mannitol plus 33.3 mL | |
| 20% = 50 mL of mannitol plus 12.5 mL | |
| 25% = – As supplied – |
For Urological Irrigation: As 2.5 percent solution is used. The use of 2.5 percent mannitol solution minimizes the hemolytic effect of water alone, the entrance of hemolyzed blood into the circulation, and the resulting hemoglobinemia which is considered a major factor in producing serious renal complications.
HOW SUPPLIED
Mannitol Injection, USP 25% (12.5 g/50 mL)
0517-4050-25 50 mL Single Dose Vial packaged in boxes of 25
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature).
IN4050
Rev. 1/09
MG # 14063
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
| MANNITOL
mannitol injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - American Regent, Inc. (002033710) |
