SCIENCEWARE EYEWASH- water solution
Bel-Art Products
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Scienceware Eyewash®
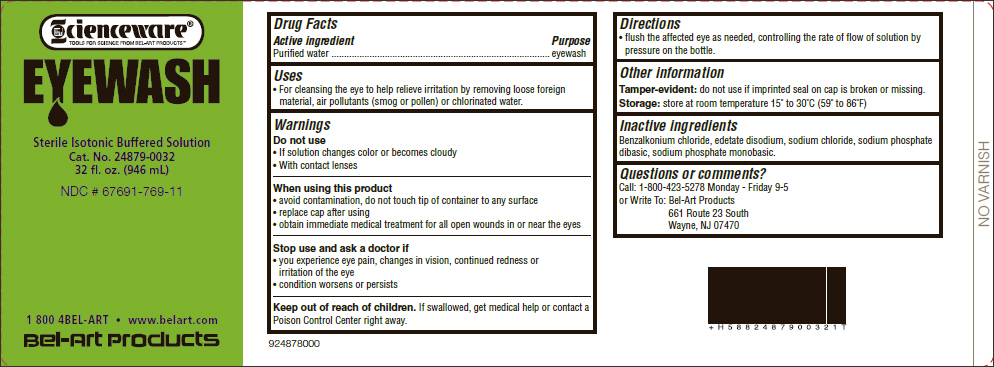
Uses
- For cleansing the eye to help relieve irritation by removing loose foreign material, air pollutants (smog or pollen) or chlorinated water.
Warnings
When using this product
- avoid contamination, do not touch tip of container to any surface
- replace cap after using
- obtain immediate medical treatment for all open wounds in or near the eyes
Directions
- flush the affected eye as needed, controlling the rate of flow of solution by pressure on the bottle.
Other information
Tamper-evident: do not use if imprinted seal on cap is broken or missing.
Storage: store at room temperature 15˚ to 30˚C (59˚ to 86˚F)
Inactive ingredients
Benzalkonium chloride, edetate disodium, sodium chloride, sodium phosphate dibasic, sodium phosphate monobasic.
| SCIENCEWARE EYEWASH
water solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bel-Art Products (002176733) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Altaire Pharmaceuticals, Inc. | 786790378 | MANUFACTURE(67691-769) | |
Revised: 2/2017
Document Id: decaf0f9-ef6f-4c90-aaaf-775c2b2ee504
Set id: 74881703-830f-4d1b-88b5-433db010f62a
Version: 2
Effective Time: 20170203
Bel-Art Products