PERDIEM- sennosides, stimulant laxative pill
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
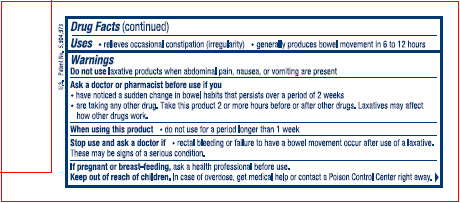
Uses
● relieves occasional constipation (irregularity)
● generally produces bowel movement in 6 to 12 hours
Warnings
Ask a doctor or pharmacist before use if you
● have noticed a sudden change in bowel habits that persists over a period of 2 weeks
● are taking any other drug. Take this product 2 or more hours before or after other drugs. Laxatives may affect how other drugs work.
Directions
● swallow pill(s) with a glass of water
● swallow pill(s) whole, do not crush, break or chew
|
Adults and children 12 years of age and older |
2 pills once or twice daily |
|
Children 6 to under 12 years of age |
1 pill once or twice daily |
|
Children under 6 years of age |
ask a doctor |
Other Information
● each pill contains: calcium 55mg
● sodium free
● protect from moisture
● store at controlled room temperature 20-25°C (68-77°F).
Inactive Ingredients
Acacia, alginic acid, black iron oxide, carnauba wax, colloidal silicon dioxide, D&C yellow #10 aluminum lake, dibasic calcium phosphate, magnesium stearate, methylparaben, microcrystalline cellulose, povidone, pregelatinized starch, propylene glycol, propylparaben, red iron oxide, shellac, sodium benzoate, sodium hydroxide, sodium lauryl sulfate, stearic acid, sucrose, talc, titanium dioxide, yellow iron oxide
| PERDIEM
sennosides, stimulant laxative pill |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |