FLOR-OPAL SUSTAINED-RELEASE FLUORIDE- sodium fluoride gel
Ultradent Products, Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Flor-Opal®
Sustained Released Fluoride Gel
Description
Flor-Opal® is a unique, sustained-release, 0.5% fluoride ion in a sticky, viscous gel to be used with a custom tray. Flor-Opal is a clear, nearly tasteless, high-viscosity sticky gel with a pH of ~6.5.
Indications
Flor-Opal is designed for use whenever topical fluoride application is desired via take-home tray (e.g., prevention of root caries, treatment of periodontal conditions, following bleaching procedures, root sensitivity).
Procedure
|
||
|  |
|
|
||
|
||
|  Fig. 1 | 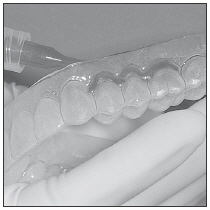 Fig. 2 |
Laboratory Instructions
- 1.
- Pour and trim cast as you would for a standard bleaching tray. Apply 0.5-1.0mm of Ultradent® LC Block-Out Resin to a clean, dry cast.
- 2.
- Cure LC Block-Out for ~2 minutes in a light curing unit. A hand-held intraoral light (VALO® or another high quality curing light (output > 600mw/cm2)) can be used. Light cure 20 seconds using a scanning motion over the arch. Carefully check resin cure by tapping the surface of the resin with an instrument. Wipe off oxygen inhibition layer.
- 3.
- With vacuum former (Ultra-Form® or EconoForm™), heat a Sof-Tray® sheet until it sags ~1 inch. Activate vacuum and adapt softened plastic over mold. Cool and remove model.
- 4.
- Cut excess bulk of material away with Ultra-Trim™ scalloping scissors.
- 5.
- Trim excess tray material. Contour the tray margin to avoid frenum attachments, tori, and similar anatomical structures.
- 6.
- Return tray to model; check tray extensions. Gently flame polish edges one quadrant at a time (Blazer® Micro Torch).
- 7.
- While still warm, hold periphery of each segment firmly against model for 3 seconds with water-moistened finger. If an area is short of the desired length, gently heat and push the tray material to the desired location. If this material thins too much, a new tray should be fabricated.
Precautions
- 1.
- If any problems occur, have the patient discontinue treatment and notify the dentist.
- 2.
- Patients should not swallow large volumes of Flor-Opal gel. Excess at insertion and residue upon removal should be brushed off and rinsed; DO NOT swallow.
- 3.
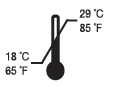
- Keep out of heat and sunlight at all times. Flor-Opal should be stored at room temperature.
- 4.
- Not intended for use by pregnant women.
EN- DENTIST INSTRUCTIONS & LAB INSTRUCTIONS
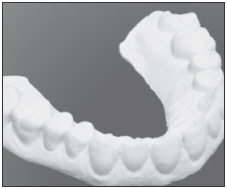 | Construct and dry the stone model, cutting palate and tongue areas out. |
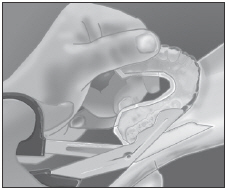 | Vacuum adapt Sof-Tray® .035˝ sheet to model. Cool and separate. |
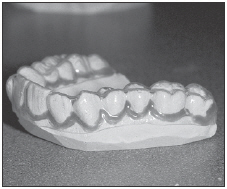 | For sensitive roots use Ultradent's LC Block-Out Resin on gingival margin to provide a reservoir space for Flor-Opal. It is recommended to lap tray 2-3mm onto gingiva. |
General Precautions
|
|
EN- PATIENT INSTRUCTIONS
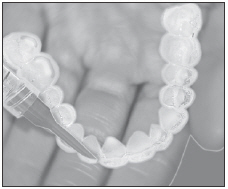 | Load gel into your bleaching tray made by your dentist. Use 1/3 to 1/2 of syringe. |
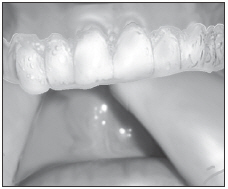 | Brush teeth, then insert tray. Adapt tray sides to teeth. |
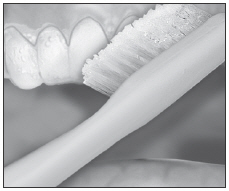 | Remove excess gel with clean finger or soft toothbrush. Rinse twice; do not swallow rinses. |
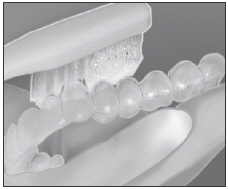 | Clean tray with soft brush and cool tap water. Store tray in case provided. |
|
| Use by date | ||
|
| See instructions | ||
|
| Recycle | ||
|
| Lot Number | ||
|
| Keep out of reach of children | ||
|
| Store at room temperature | ||
|
| Health Hazard |
| EN For Professional use only. |
Not for injection.
Ultradent syringes have an expiration date stamped on the side of the syringe consisting of one letter and three numbers.
The letter is a lot number used for manufacturing purposes and the three numbers are the expiration date.
The first two numbers are the month, and the third number is the last number of the year.
Flor-Opal
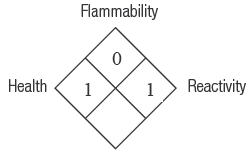 For product SDS please see our website: www.ultradent.com 1-800-552-5512; 801-572-4200 | Hazard Rating 4 = Severe 3 = Serious 2 = Moderate 1 = Slight 0 = Minimal |
For professional use only.
U.S. federal law restricts the sale of this device by or on the order of a dentist.
Keep out of reach of children.
For immediate reorder and/or complete descriptions of Ultradent's product line, refer to Ultradent's catalog or call Toll Free 1-800-552-5512.
Outside U.S. call (801) 572-4200
or visit www.ultradent.com.
© Copyright 2017 Ultradent Products, Inc. All Rights Reserved.
ULTRADENT
PRODUCTS, INC.
Manufatured by Ultradent Products, Inc.
505 West Ultradent Drive (10200 South)
South Jordan, Utah 84095, USA Made in USA
Ultradent Products GmbH
Am Westhover Berg 30
51149 Cologne Germany
10115.10 040517
| FLOR-OPAL SUSTAINED-RELEASE FLUORIDE
sodium fluoride gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Ultradent Products, Inc (013369913) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ultradent Products, Inc | 013369913 | MANUFACTURE(51206-401) , LABEL(51206-401) , ANALYSIS(51206-401) , PACK(51206-401) | |