Label: CVS PHARMACY CHILDRENS ALLERGY RELIEF- cetirizine hydrochloride solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 59779-276-01, 59779-276-04, 59779-276-08 - Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 12, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 5 mL teaspoonful)
- Purpose
- Uses
-
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
-
Directions
- use only with enclosed dosing cup
adults and children 6 years and over 1 teaspoonful (5 mL) or 2 teaspoonfuls (10 mL) once daily depending upon severity of symptoms; do not take more than 2 teaspoonfuls (10 mL) in 24 hours. adults 65 years and older 1 teaspoonful (5 mL) once daily; do not take more than 1 teaspoonful (5 mL) in 24 hours. children 2 to under 6 years of age ½ teaspoonful (2.5 mL) once daily. If needed, dose can be increased to a maximum of 1 teaspoonful (5 mL) once daily or ½ teaspoonful (2.5 mL) every 12 hours. Do not give more than 1 teaspoonful (5 mL) in 24 hours. children under 2 years of age ask a doctor consumers with liver or kidney disease ask a doctor - Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
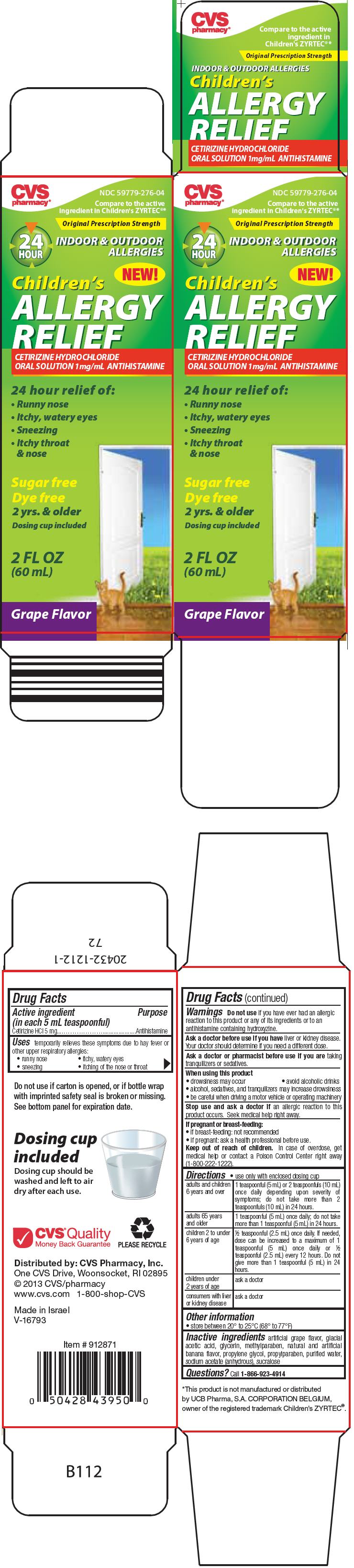
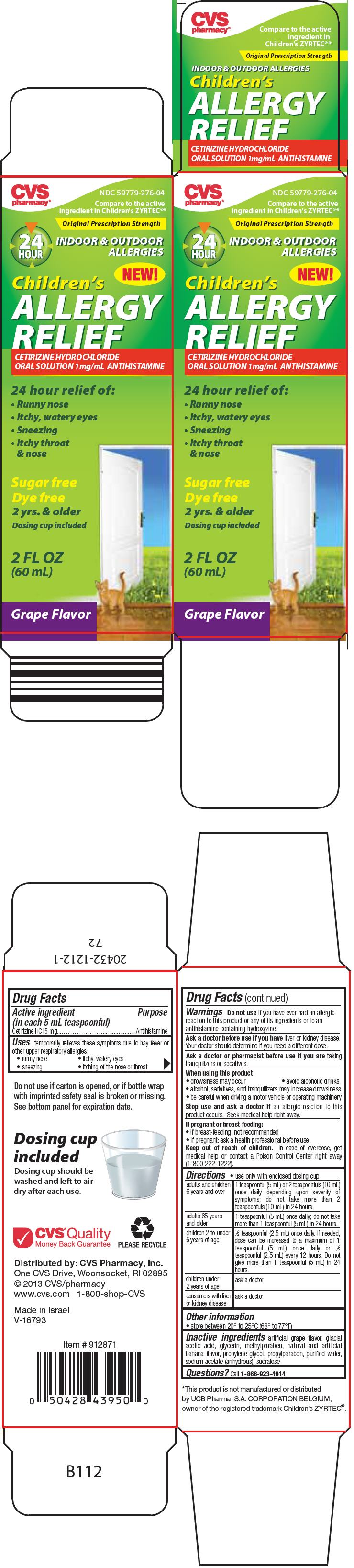
PRINCIPAL DISPLAY PANEL - 60 mL Bottle Carton
CVS
pharmacy®NDC 59779-276-04
Compare to the active
ingredient in Children's ZYRTEC®*Original Prescription Strength
24
HOURINDOOR & OUTDOOR
ALLERGIESNEW!
Children's
ALLERGY
RELIEFCETIRIZINE HYDROCHLORIDE
ORAL SOLUTION 1mg/mL ANTIHISTAMINE24 hour relief of:
- Runny nose
- Itchy, watery eyes
- Sneezing
-
Itchy throat
& nose
Sugar free
Dye free
2 yrs. & older
Dosing cup included2 FL OZ
(60 mL)Grape Flavor
-
INGREDIENTS AND APPEARANCE
CVS PHARMACY CHILDRENS ALLERGY RELIEF
cetirizine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-276 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cetirizine Hydrochloride (UNII: 64O047KTOA) (Cetirizine - UNII:YO7261ME24) Cetirizine Hydrochloride 5 mg in 5 mL Inactive Ingredients Ingredient Name Strength acetic acid (UNII: Q40Q9N063P) glycerin (UNII: PDC6A3C0OX) methylparaben (UNII: A2I8C7HI9T) propylene glycol (UNII: 6DC9Q167V3) propylparaben (UNII: Z8IX2SC1OH) water (UNII: 059QF0KO0R) sucralose (UNII: 96K6UQ3ZD4) Product Characteristics Color YELLOW (colorless to slightly yellow) Score Shape Size Flavor GRAPE (sugar free) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-276-04 1 in 1 CARTON 1 60 mL in 1 BOTTLE 2 NDC:59779-276-01 1 in 1 CARTON 2 240 mL in 1 BOTTLE 3 NDC:59779-276-08 1 in 1 CARTON 3 120 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090182 09/08/2011 Labeler - CVS Pharmacy (062312574) Registrant - Taro Pharmaceuticals U.S.A., Inc. (145186370) Establishment Name Address ID/FEI Business Operations Taro Pharmaceutical Industries Ltd. 600072078 MANUFACTURE(59779-276) Establishment Name Address ID/FEI Business Operations Taro Pharmaceticals Inc. 206263295 MANUFACTURE(59779-276)