Label: BACITRACIN ointment
- NDC Code(s): 0574-4022-01, 0574-4022-11, 0574-4022-13, 0574-4022-35
- Packager: Padagis US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 16, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- STERILE Rx Only
- DESCRIPTION:
- CLINICAL PHARMACOLOGY:
- INDICATIONS AND USAGE:
- CONTRAINDICATIONS:
-
PRECAUTIONS:
Bacitracin ophthalmic ointment should not be used in deep-seated ocular infections or in those that are likely to become systemic. The prolonged use of antibiotic containing preparations may result in overgrowth of nonsusceptible organisms particularly fungi. If new infections develop during treatment appropriate antibiotic or chemotherapy should be instituted.
-
ADVERSE REACTIONS:
Bacitracin has such a low incidence of allergenicity that for all practical purposes side reactions are practically non-existent. However, if such reaction should occur, therapy should be discontinued.
To report SUSPECTED ADVERSE REACTIONS, contact Perrigo at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
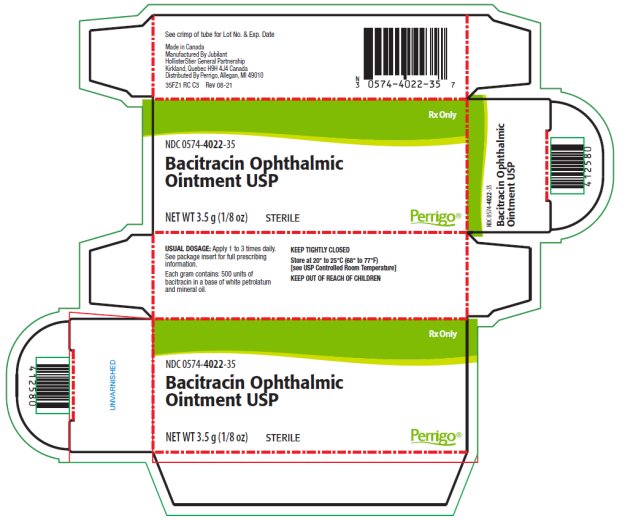
DOSAGE AND ADMINISTRATION:
The ointment should be applied directly into the conjunctival sac 1 to 3 times daily. In blepharitis all scales and crusts should be carefully removed and the ointment then spread uniformly over the lid margins. Patients should be instructed to take appropriate measures to avoid gross contamination of the ointment when applying the ointment directly to the infected eye.
- HOW SUPPLIED:
- SPL UNCLASSIFIED SECTION
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 3.5 g Carton
-
INGREDIENTS AND APPEARANCE
BACITRACIN
bacitracin ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0574-4022 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN (UNII: 58H6RWO52I) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 500 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0574-4022-01 1 in 1 CARTON 03/10/2014 09/01/2016 1 NDC:0574-4022-11 1 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0574-4022-13 3 in 1 CARTON 03/10/2014 09/01/2016 2 NDC:0574-4022-11 1 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:0574-4022-35 1 in 1 CARTON 03/10/2014 3 3.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA061212 03/10/2014 Labeler - Padagis US LLC (967694121)