Label: PRIME ASTHMA RELIEF- epinephrine capsule
PRIME ASTHMA RELIEF- epinephrine kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 15343-104-20, 15343-105-40 - Packager: DRNATURALHEALING
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 19, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
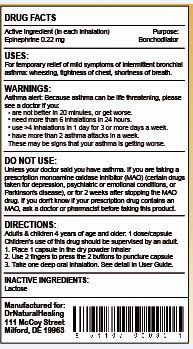
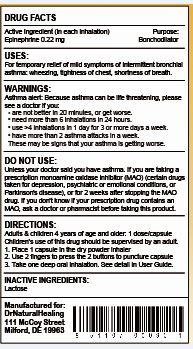
- ACTIVE INGREDIENT
- PURPOSE
- USE
-
WARNINGS
Asthma Alert:
Because asthma may be life threatening, see a doctor if you:- are not better in 20 minutes
- get worse
- need more than 6 inhalations in 24 hours
- use more than 4 inhalations in 24 hours for 3 or more days a week
- have more than 2 asthma attacks in a week
These may be signs that your asthma is getting worse
Do not use
- unless a doctor said you have asthma
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs taken for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have:
- ever been hospitalized for asthma
- heart disease
- high blood pressure
- diabetes
- thyroid disease
- seizures
- narrow angle glaucoma
- a psychiatric or emotional condition
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
- taking prescription drugs for asthma, obesity, weight control, depression, or psychiatric or emotional conditions
- taking any drug that contains phenylephrine, pseudoephedrine, ephedrine, or caffeine (such as for allergy, cough-cold, or pain)
When using this product
- your blood pressure or heart rate may go up. This could increase your risk of heart attack or stroke, which may cause death.
- Your risk of heart attack or stroke increases if you:
- have a history of high blood pressure or heart disease
- take this product more frequently or take more than the recommended dose
- avoid foods or beverages that contain caffeine
- avoid dietary supplements containing ingredients reported or claimed to have a stimulant effect
-
DIRECTIONS:
- do not use more than directed
- adults and children 4 years of age and over: 1 capsule is for one inhalation at one time
- inhalation not more often than every 3 hours
- do not use more than 12 inhalations in 24 hours
- the use of this product by children should be supervised by an adult
- children under 4 years of age: ask a doctor
1) The active ingredient in a dry powder is 0.22 mg per capsule (the concentration is 1% epinephrine in the total weight of the dry powder)
2) For use in a dry powder inhaler for holding the No. 3 capsule.
3) Should orally inhale at least two times after the capsule is pierced to make sure all dry powder is inhaled into the lung
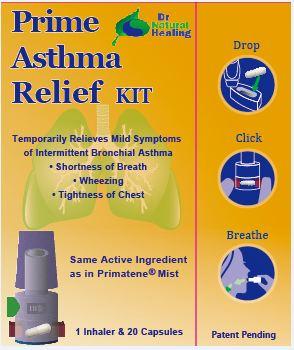
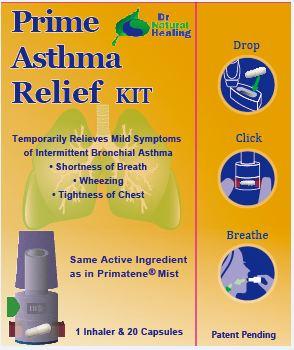
4) Instructions for Inhalation:
A. Inhaler parts:
B. Instructions for using the dry powder inhaler:
Wash and dry your hands completely before touching the inhaler and capsule.
 (1) Remove the cap.
(1) Remove the cap.
 (2) Hold the inhaler at base and turn the mouthpiece in direction of the arrow.
(2) Hold the inhaler at base and turn the mouthpiece in direction of the arrow.
 (3) Obtain 1 capsule from the blister package just prior to use.
(3) Obtain 1 capsule from the blister package just prior to use.
 (4) Open inhaler by turning mouthpiece as indicated. Load 1 capsule in the capsule-chamber of the inhaler. Do not put the capsule directly into the top of the mouthpiece. Twist the mouthpiece to the closed position.
(4) Open inhaler by turning mouthpiece as indicated. Load 1 capsule in the capsule-chamber of the inhaler. Do not put the capsule directly into the top of the mouthpiece. Twist the mouthpiece to the closed position.
 (5) Hold the inhaler upright, push/click two blue buttons inwards all the way. Let go of the blue buttons. Do not press the blue buttons more than 1 time. The chances of the capsule breaking into pieces will be increased if the capsule is accidentally pierced more than once.
(5) Hold the inhaler upright, push/click two blue buttons inwards all the way. Let go of the blue buttons. Do not press the blue buttons more than 1 time. The chances of the capsule breaking into pieces will be increased if the capsule is accidentally pierced more than once.
(6) With the inhaler away from your mouth, prepare by breathing out (exhaling) all the way. Do not blow or exhale into the mouthpiece.
 (7) Hold the inhaler horizontally. Place your mouth over the mouthpiece and close your lips tightly around it. Inhale quickly and deeply.
(7) Hold the inhaler horizontally. Place your mouth over the mouthpiece and close your lips tightly around it. Inhale quickly and deeply.
(8) Hold your breath for count of ten, or as long as is comfortable. Breathe out gently. You will need to inhale at least twice from one capsule in order to get the full dose.
(9) After the dose, open the mouthpiece, remove the used capsule, and take a look at the used capsule. It should be pierced and empty. If so, dispose of it properly. If the capsule does not look pierced, put the capsule back into the device capsule-chamber, repeat steps 7, 8 and 9.
(10) Cover the mouthpiece and dust cap for storage.
(11) After your Inhalation, throw away the empty capsule properly as it is bio-degradable. Do not store the capsule in the inhaler. Put the mouthpiece back to your inhaler device and twist the mouthpiece in a clockwise direction until it is tight. Do not over-tighten. - HOW SUPPLIED
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- REGULATORY STATUS
- COMMENTS AND QUESTIONS
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PRIME ASTHMA RELIEF
epinephrine capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:15343-105 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.22 mg Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) 49.78 mg Product Characteristics Color white Score no score Shape capsule Size 3mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:15343-105-40 1 in 1 CARTON 1 40 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/21/2014 PRIME ASTHMA RELIEF
epinephrine kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:15343-104 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:15343-104-20 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 20 Part 1 of 1 PRIME ASTHMA RELIEF
epinephrine capsuleProduct Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.22 mg Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) Product Characteristics Color white Score no score Shape capsule Size 3mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 20 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/21/2014 Labeler - DRNATURALHEALING (129613308) Registrant - DRNATURALHEALING (129613308) Establishment Name Address ID/FEI Business Operations Star Health and Beauty LLC 013506838 manufacture(15343-104)