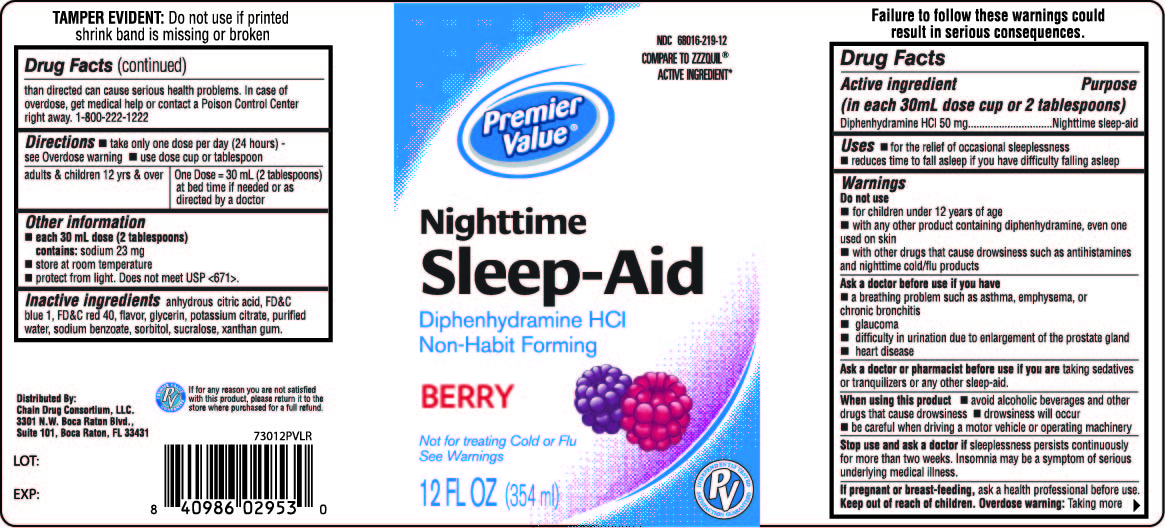
Label: PREMIER VALUE NIGHTTIME SLEEP-AID LIQUID SLEEP-AID- diphenhydramine hydrochloride liquid
- NDC Code(s): 68016-219-12
- Packager: Chain Drug Consortium
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients (in each 30 mL dose cup or 2 tablespoons)
- Purpose
- Uses
-
Warnings
Do not use
- •
- for children under 12 years of age
- •
- with any other product containing diphenhydramine, even one used on skin
- •
- with other drugs that cause drowsiness such as antihistamines and nighttime cold/flu products
Ask a doctor before use if you have
- •
- a breathing problem such as asthma, emphysema, or chronic bronchitis
- •
- glaucoma
- •
- difficulty in urination due to enlargement of the prostate gland
- •
- heart disease
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers or any other sleep aid
When using this product
- •
- avoid alcoholic beverages and other drugs that cause drowsiness
- •
- drowsiness will occur
- •
- be careful when driving a motor vehicle or operating machinery
-
Directions
- •
- take only one dose per day (24 hours) - see Overdose warning
- •
- use dose cup or tablespoon
adults & children 12 yrs & over
One Dose = 30 mL (2 tablespoons) at bed time if needed or as directed by a doctor
- Other information
- Inactive ingredients
-
SPL UNCLASSIFIED SECTION
Failure to follow these warnings could result in serious consequences
TAMPER EVIDENT: This package is safety sealed & child resistant. Use only if blisters are intact. If difficult to open, use scissors.
This product is not manufactured or distributed by Procter & Gamble, the distributor of ZzzQuil™.
Distributed by:
Chain Drug Consortium LLC.
3301 N.W. Boca Raton Blvd.
Suite 101, Boca Raton FL 33431
- PRINCIPAL DISPLAY PANEL - 354 ml Bottle Label
-
INGREDIENTS AND APPEARANCE
PREMIER VALUE NIGHTTIME SLEEP-AID LIQUID SLEEP-AID
diphenhydramine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-219 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 50 mg in 30 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM CITRATE (UNII: EE90ONI6FF) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color PURPLE Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-219-12 354 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/10/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M010 12/10/2012 Labeler - Chain Drug Consortium (101668460)