Label: NYSTATIN ointment
- NDC Code(s): 45802-048-11, 45802-048-35
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
-
CLINICAL PHARMACOLOGY
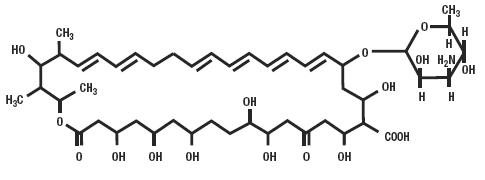
Nystatin is an antifungal antibiotic which is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi. It probably acts by binding to sterols in the cell membrane of the fungus with a resultant change in membrane permeability allowing leakage of intracellular components. Nystatin is a polyene antibiotic that is obtained from Streptomyces noursei, and is the first well tolerated antifungal antibiotic of dependable efficacy for the treatment of cutaneous, oral and intestinal infections caused by Candida [Monilia]albicans and other Candida species. It exhibits no appreciable activity against bacteria.
Nystatin Ointment USP provides specific therapy for all localized forms of candidiasis. Symptomatic relief is rapid, often occurring within 24 to 72 hours after the initiation of treatment. Cure is effected both clinically and mycologically in most cases of localized candidiasis.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- PRECAUTIONS
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
Nystatin Ointment USP should be applied liberally to affected areas twice a day or as indicated until healing is complete. Nystatin cream is usually preferred to nystatin ointment in candidiasis involving intertriginous areas; very moist lesions, however, are best treated with nystatin topical powder.
This preparation does not stain skin or mucous membranes and provides a simple, convenient means of treatment.
- HOW SUPPLIED
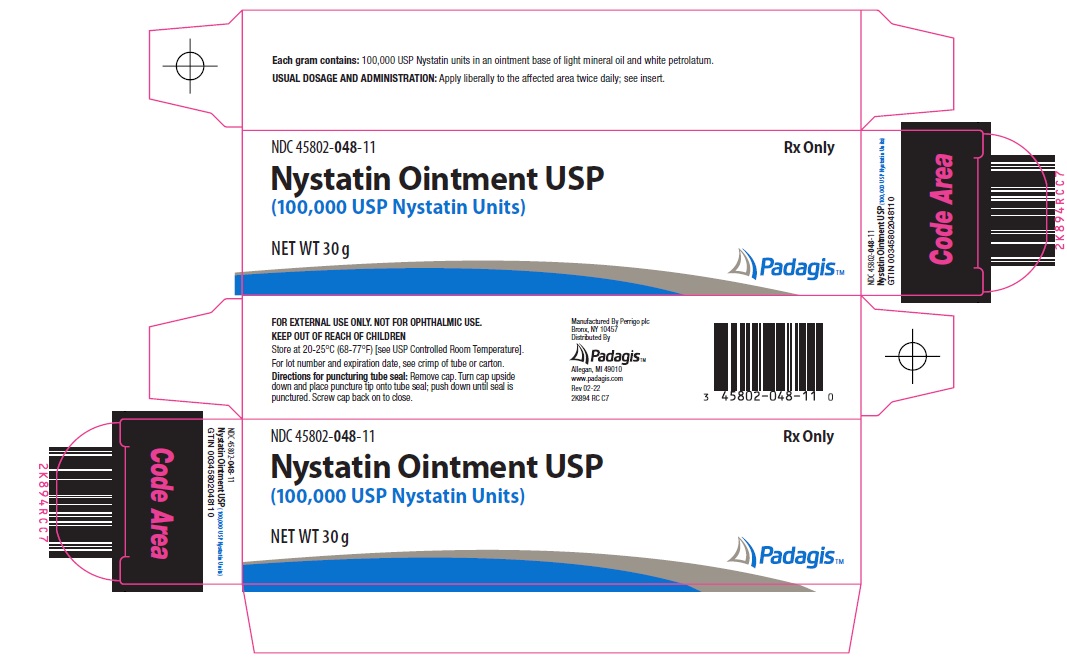
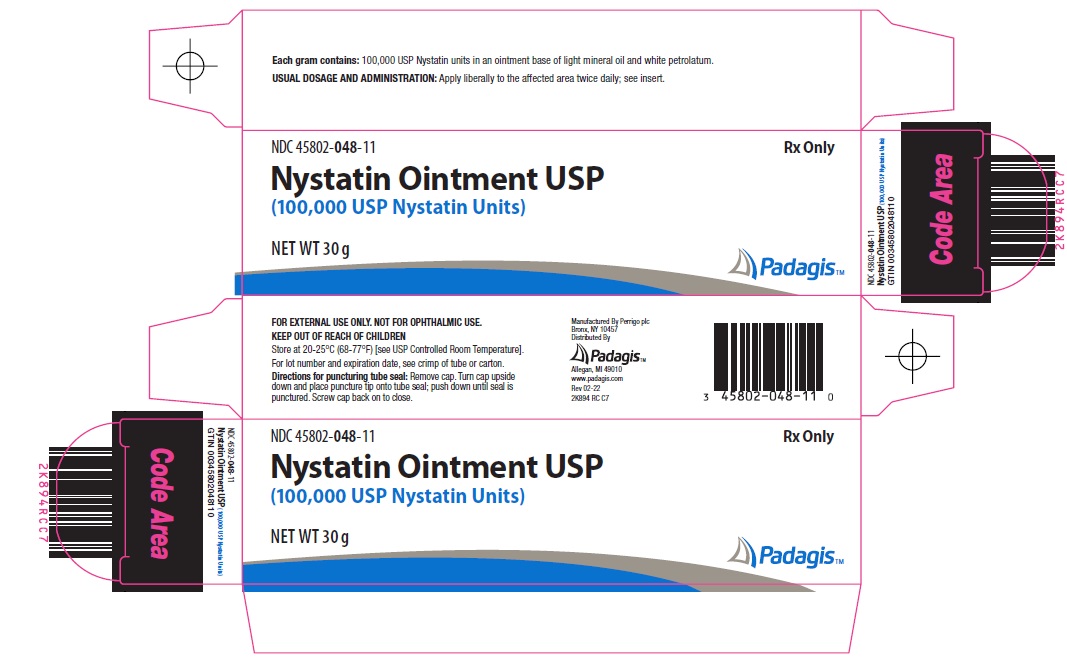
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
NYSTATIN
nystatin ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:45802-048 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NYSTATIN (UNII: BDF1O1C72E) (NYSTATIN - UNII:BDF1O1C72E) NYSTATIN 100000 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength LIGHT MINERAL OIL (UNII: N6K5787QVP) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45802-048-35 1 in 1 CARTON 09/15/2006 1 15 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:45802-048-11 1 in 1 CARTON 11/08/2006 2 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA062472 09/15/2006 Labeler - Padagis Israel Pharmaceuticals Ltd (600093611)