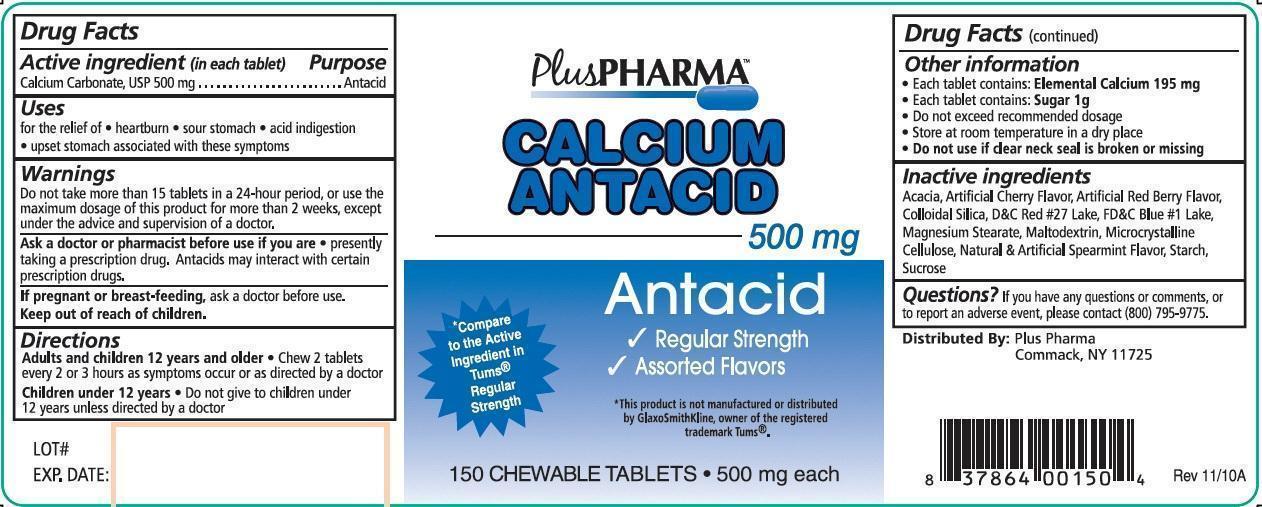
PLUS PHARMA CALCIUM ANTACID- calcium carbonate tablet, chewable
Gemini Pharmaceuticals, Inc. dba Plus Pharma
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Calcium Antacid
Uses
for the relief of
- heartburn
- sour stomach
- acid indigestion
- upset stomach associated with these symptoms
Warnings
Do not take more than 15 tablets in a 24-hour period, or use the maximum dosage of this product for more than 2 weeks, except under the advice and supervision of a doctor.
Directions
Adults and children 12 years and older
Chew 2 tablets every 2 or 3 hours as symptoms occur or as directed by a doctor
Children under 12 years
Do not give to children under 12 years unless directed by a doctor
Other information
- Each tablet contains: Elemental Calcium 195 mg
- Each tablet contains: Sugar 1g
- Do not exceed recommended dosage
- Store at room temperature in a dry place
- Do not use if clear neck seal is broken or missing
Inactive ingredients
Acacia, Artificial Cherry Flavor, Artificial Red Berry Flavor, Colloidal Silica, D and C Red #27 Lake, FD and C Blue #1 Lake, Magnesium Stearate, Maltodextrin, Microcrystalline Cellulose, Natural and Artificial Spearmint Flavor, Strach, Sucrose
Questions?
If you have any questions or comments, or to report an adverse event, please contact (800) 795-9775.
Principal Display Panel
PlusPHARMA
CALCIUM ANTACID
500 mg
Antacid
- Regular Strength
- Assorted Flavors
*Compare to the Active Ingredient in Tums® Regular Strength
*This product is not manufactured or distributed by GlaxoSmithKline, owner of the registered trademark Tums®.
150 CHEWABLE TABLETS 500 mg each
| PLUS PHARMA
CALCIUM ANTACID
calcium carbonate tablet, chewable |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Gemini Pharmaceuticals, Inc. dba Plus Pharma (055942270) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Gemini Pharmaceuticals, Inc. dba Plus Pharma | 055942270 | manufacture(51645-735) | |