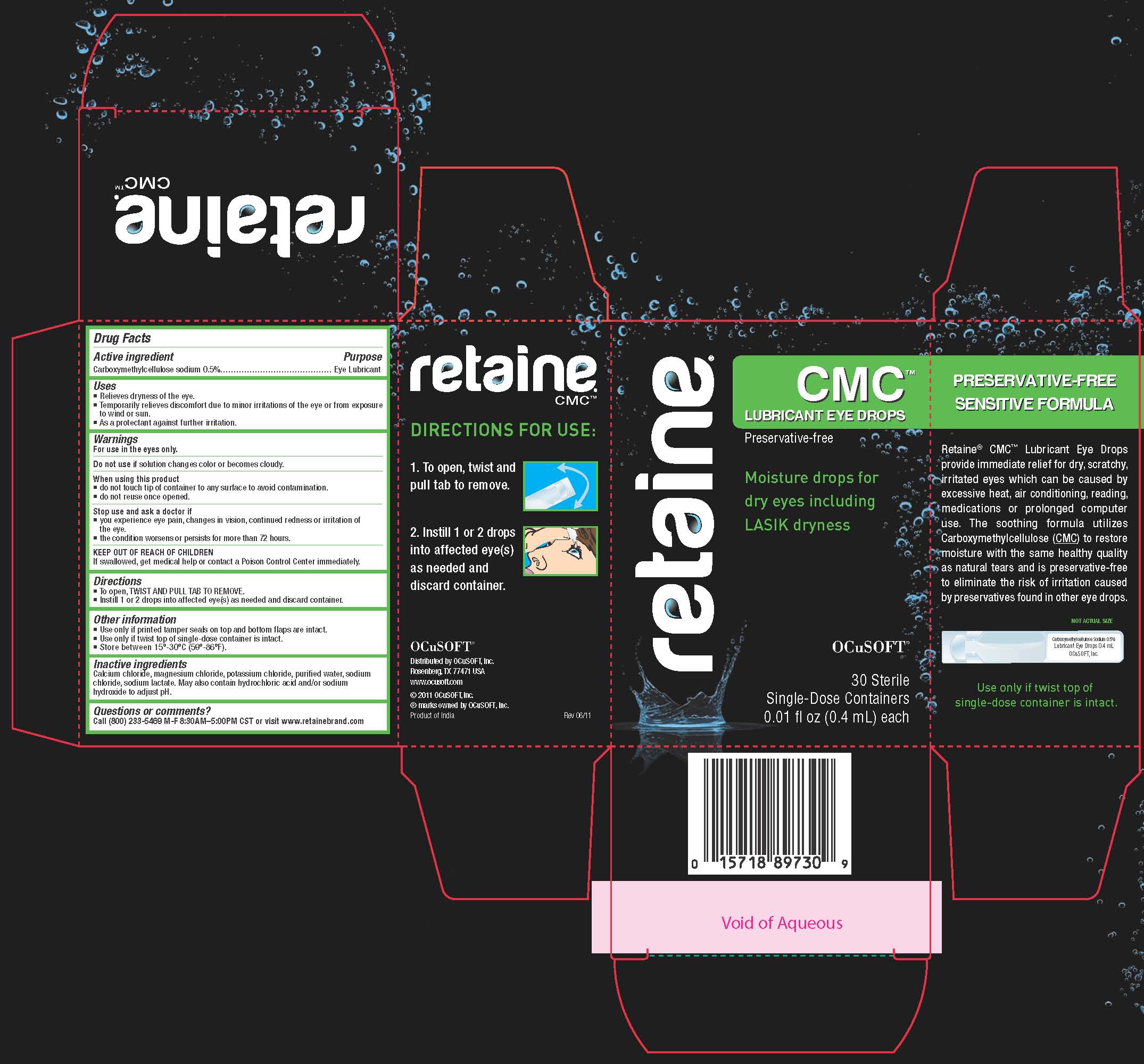
RETAINE CMC LUBRICANT EYE DROPS - carboxymethylcellulose sodium solution
OCuSOFT Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
- Relieves dryness of the eye.
- Temporarily relieves discomfort due to minor irritations of the eye or from exposure to wind or sun.
- As a protectant against further irritation.
When using this product
- do not touch tip of container to any surface to avoid contamination.
- do not reuse once opened.
Stop use and ask a doctor if
- you experience eye pain, changes in vision, continued redness or irritation of the eye.
- the condition worsens or persists for more than 72 hours.
KEEP OUT OF THE REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- To open, TWIST AND PULL TAB TO REMOVE.
- Instill 1 or 2 drops into affected eye(s) as needed and discard container.
Other Information
- Use only if printed tamper seals on top and bottom flaps are intact.
- Use only if twist top of single-dose container is intact.
- Store between 15º-30ºC (59º-86ºF).
| RETAINE CMC LUBRICANT EYE DROPS
carboxymethylcellulose sodium solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - OCuSOFT Inc. (174939207) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Altaire Pharmaceuticals, Inc. | 786790378 | manufacture(54799-897) | |
Revised: 10/2021
Document Id: 0794f6a0-f6c7-4da3-bf27-65546f2bc89c
Set id: 6b40c38e-31f8-4aca-9456-04128e6c2e93
Version: 5
Effective Time: 20211025
OCuSOFT Inc.