Label: CLORPACTIN WCS-90- chlorine powder, for solution
- NDC Code(s): 0327-0001-10
- Packager: United-Guardian, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 23, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Uses
- Warnings
-
Directions
Add the Clorpactin WCS-90 powder to sterile or deionized water at room temperature (20-25° C.). The powder dissolves slowly in water. As a result even after 2-3 minutes of stirring or mixing some residue of incompletely dissolved product will remain. This residue consists of inactive ingredients and therefore there is no necessity to continue to stir or mix for a longer period of time. This residue can be removed by either filtering the solution through a coarse laboratory filter or allowing the solution to settle for about 5 minutes and then decanting the clear solution for use.
Storage
Clorpactin WCS-90 solutions should preferably be used as soon as possible after preparation. If the solution must be stored, it can be kept refrigerated (2-8° C.) for up to 10 days in a capped or sealed plastic or glass container using a non-metallic cap. It can be stored at room temperature (20-25° C.) for up to 3 days after preparation.
Handling
As with any chlorinated product, Clorpactin WCS-90 solution should be prepared in a ventilated area and inhalation of the vapors should be minimized. The solution can cause bleaching of fabrics or other materials if splashing or spilling occurs.
- Inactive ingredients
-
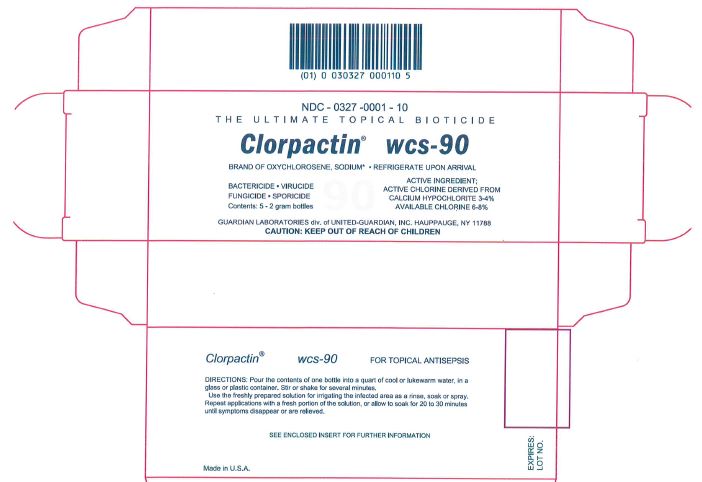
Package/Label Principal Display Panel
NDC - 0327 -0001 - 10
THE ULTIMATE TOPICAL BIOTICIDE
Clorpactin® wcs-90
BRAND OF OXYCHLOROSENE, SODIUM* • REFRIGERATE UPON ARRIVIAL
BACTERICIDE • VIRUCIDE
FUNGICIDE • SPORICIDE
Contents: 5 - 2 gram bottlesACTIVE INGREDIENT:
ACTIVE CHLORINE DERIVED FROM
CALCIUM HYPOCHLORITE 3-4%
AVAILABLE CHLORINE 6-8%GUARDIAN LABORATORIES div. of UNITED-GUARDIAN, INC. HAUPPAUGE, NY 11788
CAUTION: KEEP OUT OF REACH OF CHILDREN
Clorpactin® wcs-90 FOR TOPICAL ANTISEPSIS
DIRECTIONS: Pour the contents of one bottle into a quart of cool or lukewarm water, in a
glass or plastic container. Stir or shake for several minutes.
Use the freshly prepared solution for irrigating the infected area as a rinse, soak or spray.
Repeat applications with a fresh portion of the solution, or allow to soak for 20 to 30 minutes until symptoms disappear or are relieved.SEE ENCLOSED INSERT FOR FURTHER INFORMATION
Made in U.S.A.
Box Label
-
INGREDIENTS AND APPEARANCE
CLORPACTIN WCS-90
chlorine powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0327-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORINE (UNII: 4R7X1O2820) (CHLORINE - UNII:4R7X1O2820) CHLORINE 0.16 g in 2 g Inactive Ingredients Ingredient Name Strength SODIUM ACID PYROPHOSPHATE (UNII: H5WVD9LZUD) SODIUM POLYMETAPHOSPHATE (UNII: P1BM4ZH95L) SODIUM DODECYLBENZENESULFONATE (UNII: 554127163Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0327-0001-10 5 in 1 BOX 01/01/1955 1 2 g in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 01/01/1955 Labeler - United-Guardian, Inc. (050594555) Establishment Name Address ID/FEI Business Operations United-Guardian, Inc. 050594555 MANUFACTURE(0327-0001)