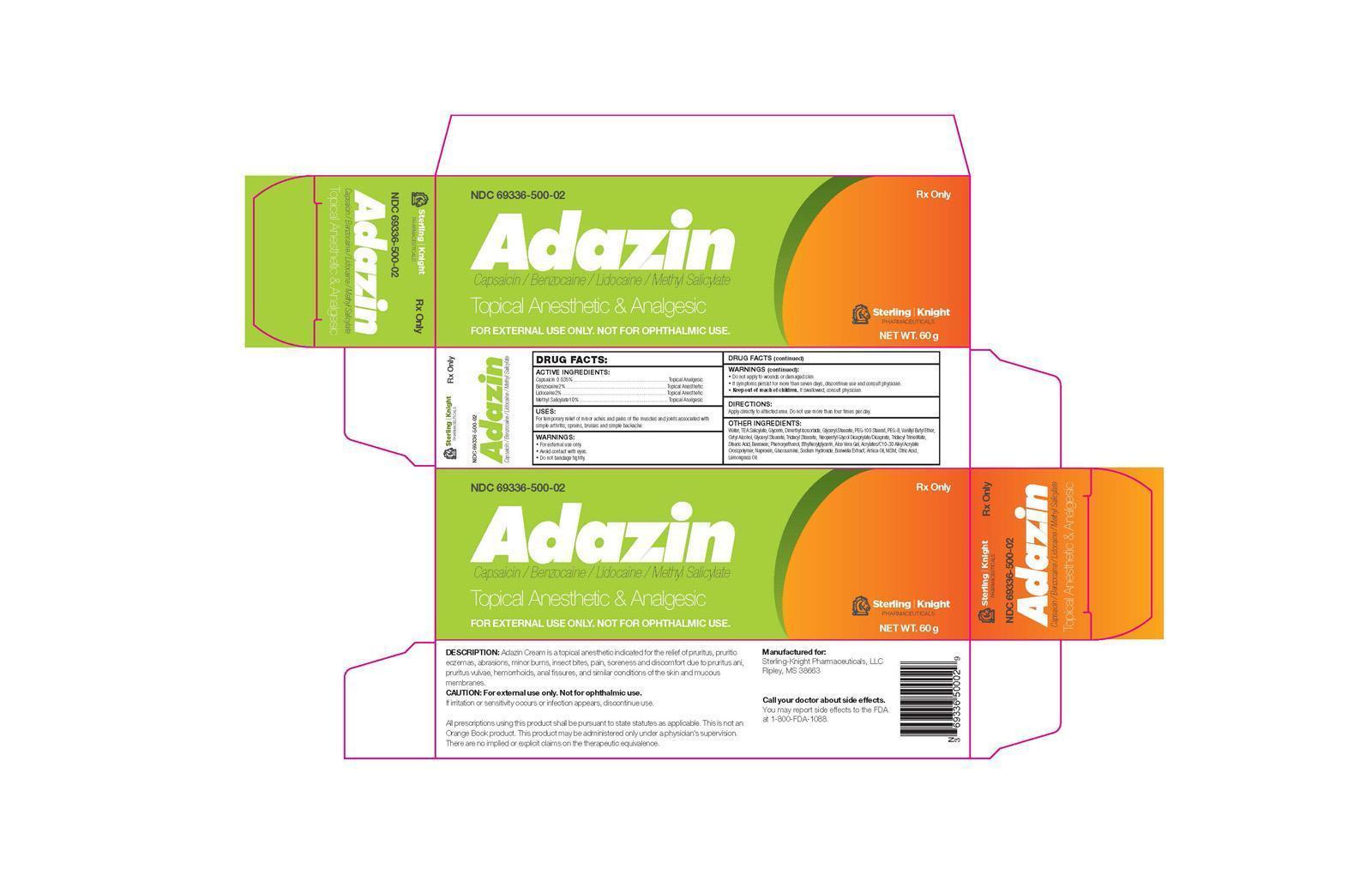
ADAZIN- capsaicin/benzocaine/lidocaine/methyl salicylate cream
Sterling Knight Pharmaceuticals, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Adazin
Rx Only
Description
Adazin is a Capsaicin 0.035% and Benzocaine 2% and Lidocaine 2% and Methyl Salicylate 10% Cream is a topical anesthetic and analgesic indicated for the relief of pruritus, pruritic eczemas, abrasions, minor burns, insect bites, pain, soreness, and discomfort due to pruritus ani, pruritus vulvae, hemorrhoids, anal fissures, and similar conditions of the skin and mucous membranes.
Active Ingredients
Each gram of Adazin Cream contains:
Capsaicin 0.035% (0.35mg) Topical Analgesic
Benzocaine 2% (20mg) Topical Anesthetic
Lidocaine 2% (20mg) Topical Anesthetic
Methyl Salicylate 10% (100mg) Topical Analgesic
Inactive Ingredients
Water, TEA Salicylate, Glycerin, Dimethyl Isosorbide, Glyceryl Stearate, PEG-100 Stearat, PEG-8, Vanillyl Butyl Ether, Cetyl Alcohol, Glyceryl Stearate, Tridecyl Stearate, Neopentyl Glycol Dicaprylate/Dicaprate, Tridecyl Trimellitate, Stearic Acid, Beeswax, Phenoxyethanol, Ethylhexylglycerin, Aloe Vera Gel, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Naproxen, Glucosamine, Sodium Hydroxide, Boswelia Extract, Arnica Oil, MSM, Citric Acid, Lemongrass Oil.
Indications
Anesthetic for relief of pruritus, pruritic eczemas, abrasions, minor burns, insect bites, pain, soreness and discomfort due to pruritus ani, pruritus vulvae, hemorrhoids, anal fissures, and similar conditions of the skin and mucous membranes.
Contraindications
Traumatized mucosa, secondary bacterial infection of the area of proposed application and known hypersensitivity to any of the components. Lidocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type.
Precautions
If irritation or sensitivity occurs or infection appears, discontinue use and institute appropriate therapy. Adazin Cream should be used with caution in ill, elderly, debilitated patients and children who may be more sensitive to the systemic effects of lidocaine.
Carcinogenesis, Mutagenesis, and Impairment of Fertility:
Studies of lidocaine in animals to evaluate the carcinogenic and mutagenic potential of the effect on fertility have not been conducted.
USE IN PREGNANCY:
Teratogenic Effects; Pregnancy Category B.
Reproduction studies have been performed for lidocaine in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to the fetus caused by lidocaine. There are, however, no adequate and well-controlled studies in pregnant women. Animal reproduction studies are not always predictive of human response. General consideration should be given to this fact before administering lidocaine to women of childbearing potential, especially during early pregnancy when maximum organogenesis takes place.
NURSING MOTHERS:
Lidocaine is excreted in human milk. The clinical significance of this observation is unknown. Caution should be exercised when lidocaine is administered to a nursing woman.
PEDIATRIC USE:
Dosage in pediatric patients should be reduced commensurate with age, body weight and physical condition.
ADVERSE REACTIONS:
During or immediately after treatment, the skin at the site of treatment may develop erythema or edema or may be the locus of abnormal sensation.
CALL YOUR DOCTOR ABOUT SIDE EFFECTS.
Call your doctor about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
DOSAGE AND ADMINISTRATION:
Apply a thin film to the affected area two or three times daily or as directed by a physician.
KEEP THIS AND ALL MEDICATIONS OUT OF REACH OF CHILDREN.
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. This product may be administered only under a physician’s supervision. There are no implied or explicit claims on the therapeutic equivalence.
Store at 25ºC (77ºF); excursions permitted to 15º-30ºC (59º-86º F). See USP Controlled Room Temperature. Protect from freezing.
Manufactured for:
Sterling-Knight Pharmaceuticals, LLC
Ripley, MS 38663
Item: 50002
Rev. 11/14
| ADAZIN
capsaicin/benzocaine/lidocaine/methyl salicylate cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Sterling Knight Pharmaceuticals, LLC (079556942) |