PANTENE PRO-V EXPERT COLLECTION HAIR REGROWTH TREATMENT FOR WOMEN- minoxidil liquid
The Procter & Gamble Manufacturing Company
----------
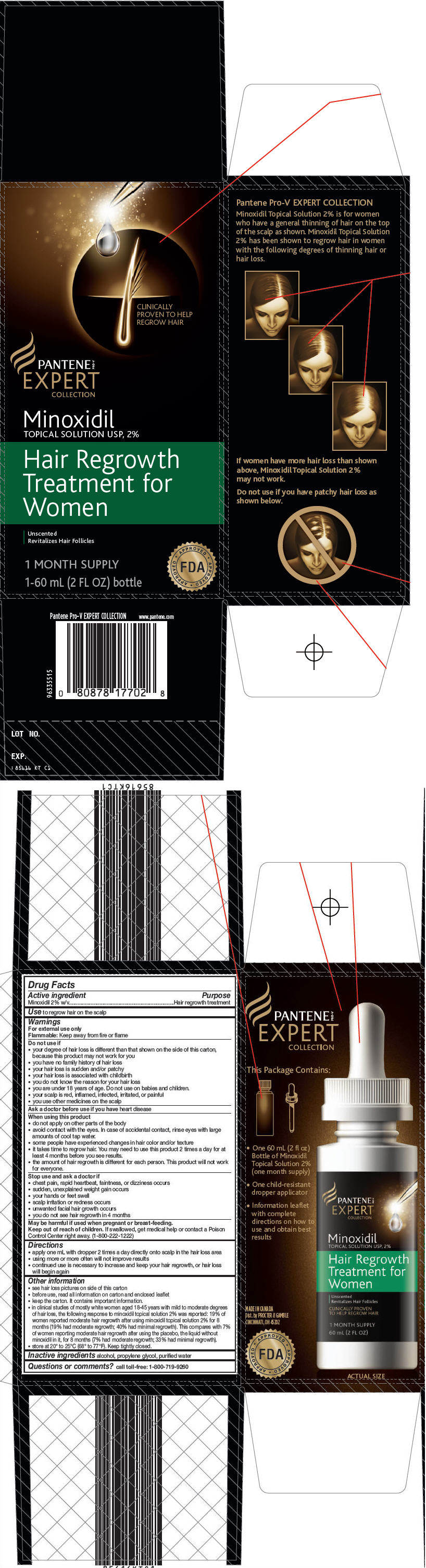
Pantene Pro-V Expert Collection
Hair Regrowth Treatment for Women
Warnings
For external use only
Flammable: Keep away from fire or flame
Do not use if
- your degree of hair loss is different than that shown on the side of this carton, because this product may not work for you
- you have no family history of hair loss
- your hair loss is sudden and/or patchy
- your hair loss is associated with childbirth
- you do not know the reason for your hair loss
- you are under 18 years of age. Do not use on babies and children.
- your scalp is red, inflamed, infected, irritated, or painful
- you use other medicines on the scalp
When using this product
- do not apply on other parts of the body
- avoid contact with the eyes. In case of accidental contact, rinse eyes with large amounts of cool tap water.
- some people have experienced changes in hair color and/or texture
- it takes time to regrow hair. You may need to use this product 2 times a day for at least 4 months before you see results.
- the amount of hair regrowth is different for each person. This product will not work for everyone.
Directions
- apply one mL with dropper 2 times a day directly onto scalp in the hair loss area
- using more or more often will not improve results
- continued use is necessary to increase and keep your hair regrowth, or hair loss will begin again
Other information
- see hair loss pictures on side of this carton
- before use, read all information on carton and enclosed leaflet
- keep the carton. It contains important information.
- in clinical studies of mostly white women aged 18-45 years with mild to moderate degrees of hair loss, the following response to minoxidil topical solution 2% was reported: 19% of women reported moderate hair regrowth after using minoxidil topical solution 2% for 8 months (19% had moderate regrowth; 40% had minimal regrowth). This compares with 7% of women reporting moderate hair regrowth after using the placebo, the liquid without minoxidil in it, for 8 months (7% had moderate regrowth; 33% had minimal regrowth).
- store at 20° to 25°C (68° to 77°F). Keep tightly closed.
| PANTENE PRO-V EXPERT COLLECTION
HAIR REGROWTH TREATMENT FOR WOMEN
minoxidil liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |
Revised: 3/2017
Document Id: 4adf4a34-d12f-397a-e054-00144ff8d46c
Set id: 6a5e6d79-0817-45ce-a7fe-e30a45414093
Version: 4
Effective Time: 20170316
The Procter & Gamble Manufacturing Company